
Interpretation:
The way the given compound is prepared using
Concept Introduction:
Ether are compounds that contain an oxygen atom that is sandwiched between two R groups. The R groups may be alkyl, vinyl or aryl.
Alcohols when treated with a very strong base undergoes deprotonation to form an alkoxide. The formed alkoxide ions can acts as a strong nucleophile for the
The general scheme for Williamson ether synthesis can be given as,
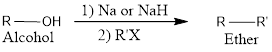
Answer to Problem 13.70P
The reaction scheme can be given as,
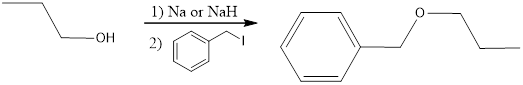
Explanation of Solution
Given compound is,
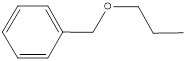
First step is to analyse the
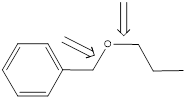
In the problem statement it is given that the alcohol is 1-propanol. Hence, the other
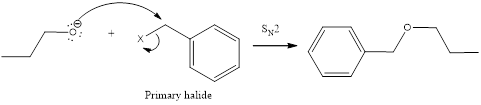
The following reaction sequence can be considered for obtaining the given compound via Williamson ether synthesis using 1-propanol.
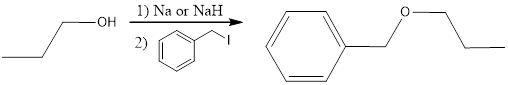
The primary halide considered here is an iodide. This is because alkyl iodides are more reactive than chlorides and bromides.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry As a Second Language: First Semester Topics
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





