
Produce the SLE phase diagram for the p-dichlorobenzene (1) + p-dibromobenzene system at 1 atm. You will do the modeling in two ways and answer each part of the question. Some helpful data are provided in Table P13-30a:
TABLE P13-30a Relevant pure component data for p-dichlorobenzene and p-dibromobenzene at 1 atm.
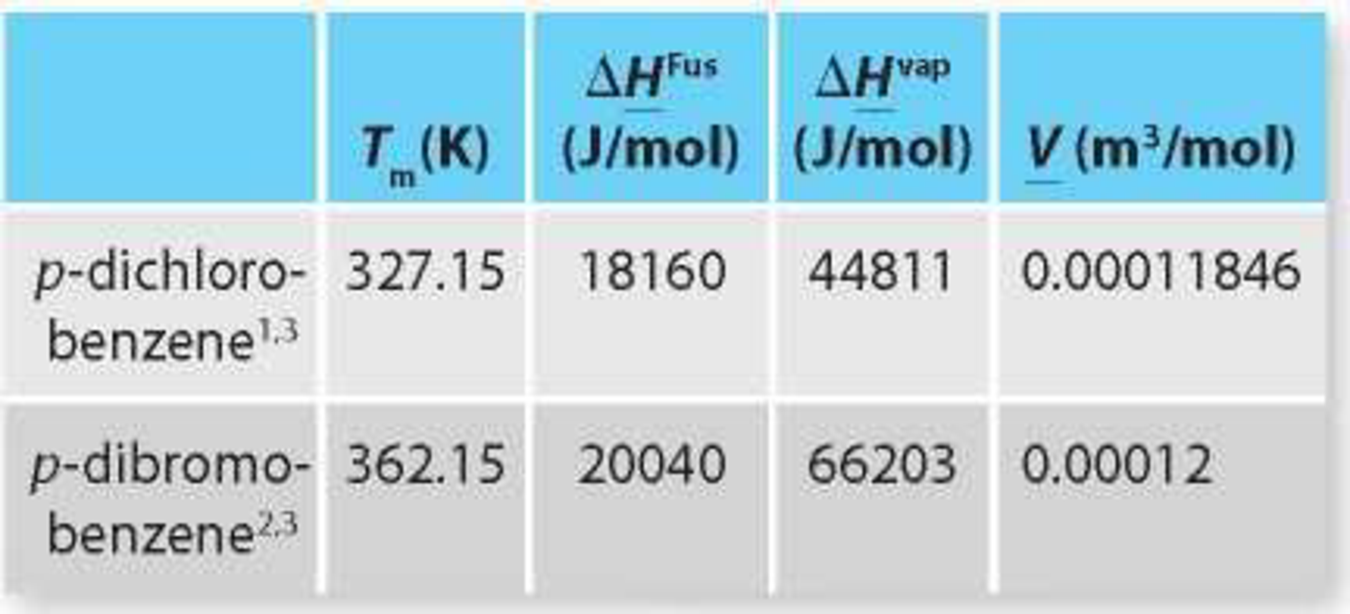
1 Heat of melting and molar volume data are from Yaws, 2003.
2 Heat of melting data is from the NIST Webbook (Linstrom and Mallard, 2012). Molar volume data are from Hildebrand, 1919.
3 Enthalpy of vaporization data are estimated using the Antoine equation from the NIST Webbook and the Clausius-Clapeyron equation.
- A. Treat the liquid phase as an ideal solution and the solid phase as immiscible. Please plot your phase diagram using the p-dichlorobenzene as the independent variable. What is the eutectic temperature and composition from the model? Compare your work with the experimental data provided in Table P13-30b.
- B. Treat the liquid phase as described by regular solution theory using the Scatchard-Hildebrand approach and the solid phase as immiscible. Please plot your phase diagram using the p-dichlorobenzene as the independent variable. What is the eutectic temperature and composition from this model? Compare your work with the experimental data provided in Table P13-30b.
- C. If you have a liquid mixture that is 76% p-dichlorobenzene and cool it until you meet the liquidus line, what is the composition of the solid precipitate and the temperature at which this occurs for both models? How does this compare to the experimental result?
TABLE P13-30b Solid-liquid equilibrium for the p-dichlorobenzene + p-dibromobenzene system at 1 atm.
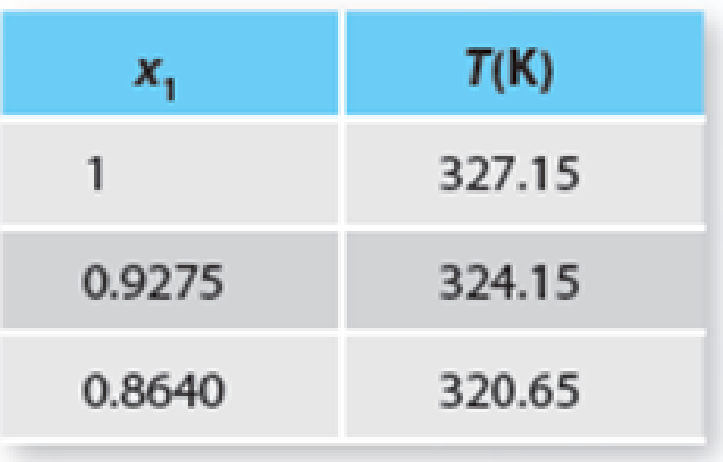
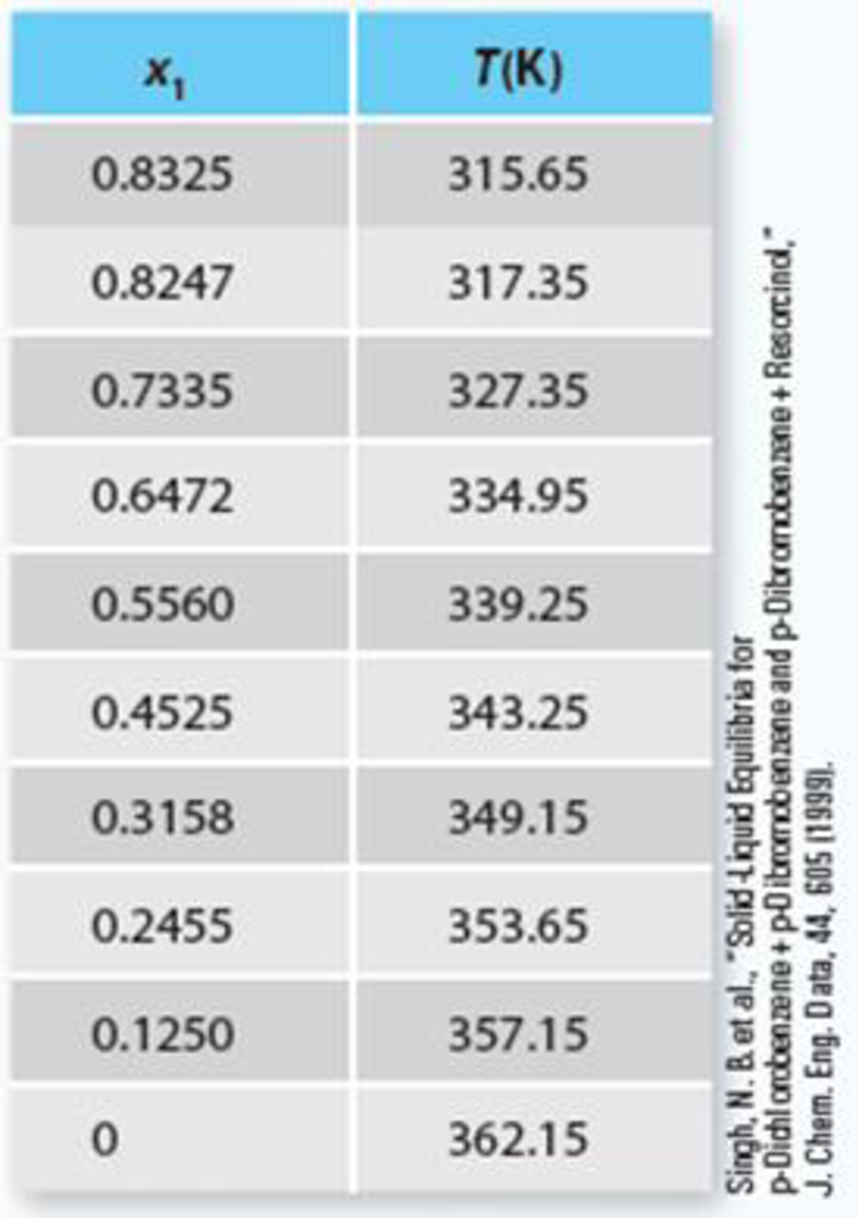
Trending nowThis is a popular solution!

Chapter 13 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





