
Concept explainers
An insulated tank that contains 1 kg of O2 at 15°C and 300 kPa is connected to a 2-m3 uninsulated tank that contains N2 at 50°C and 500 kPa. The valve connecting the two tanks is opened, and the two gases form a homogeneous mixture at 25°C. Determine (a) the final pressure in the tank, (b) the heat transfer, and (c) the entropy generated during this process. Assume T0 = 25°C.
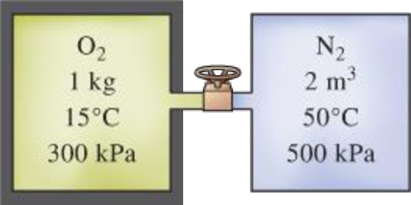
FIGURE P13–56
(a)
The pressure of the mixture.
Answer to Problem 56P
The pressure of the mixture is
Explanation of Solution
Refer to Table A-2, obtain the constant-volume specific heats of the gases at room temperature.
Write the equation to calculate the volume of the oxygen tank.
Here, mass of oxygen tank is
Calculate the mass of nitrogen gas.
Here, initial temperature and pressure of nitrogen gas is
Calculate the total volume.
Calculate the mole numbers of
Here, molar mass of
Calculate the mole number of the mixture.
Calculate the pressure of the mixture.
Here, universal gas constant of the mixture is
Conclusion:
Refer to Table A-1, obtain the gas constants of
Substitute 1 kg for
Substitute
Substitute
Refer to Table A-1, obtain the molar mass of
Substitute 1 kg for
Substitute 10.43 kg for
Substitute
Substitute
Thus, the pressure of the mixture is
(b)
The heat transfer.
Answer to Problem 56P
The heat transfer is
Explanation of Solution
Write the equation of energy balance for a closed system.
Here, heat output is
Conclusion:
Substitute 1 kg for
Thus, the heat transfer is
(c)
The entropy generation.
Answer to Problem 56P
The entropy generation is
Explanation of Solution
Write the equation of entropy balance.
Here, entropy at inlet and exit is
Calculate the mole fraction of
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Calculate the value of
Here, the partial pressure of mixture at state 2 is
Conclusion:
Substitute
Substitute
Refer to Table A-2, obtain the constant-pressure specific heats of the gases at room temperature.
Substitute 0.077 for
Substitute 0.923 for
Substitute
Thus, the entropy generation is
Want to see more full solutions like this?
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
- Argon gas enters an adiabatic turbine at 800°C and 1.5 MPa at a rate of 80 kg/min and exhausts at 200 kPa. If the power output of the turbine is 370 kW, determine the isentropic efficiency of the turbinearrow_forward10 kg of saturated liquid R-134a at 800 kPa is brought in contact with 2 kg ofsaturated vapor R-134a at the same pressure. Determine if any phase change will occur and why? How about if the vapor is superheated at the same pressure and at T = 50 ◦C?arrow_forwardWhat is the change in entropy when 120 g of superheated methanol gas at 1.00 bar and 110.0 °C is cooled and condensed to liquid methanol at 1.00 bar and 90.5 °C?arrow_forward
- Determine the reversible work in kJ when 6 moles of a gas expanded reversibly from 5 ft³ to 8 ft³ at 400K.arrow_forwardWhat is the difference between entropies of oxygen at 150 kPa and 39°C and oxygen at 150 kPa and 337°C on a perunit-mass basis?arrow_forwardA 50kg copper block initially at 180 degrees C is dropped into an isolated tank containing 90 L of water at 15 degrees C. Determine the final equilibrium temperature and the total entropy change for the process.arrow_forward
- A certain polyatomic gas stored at a 180-L rigid tank and 11 atm is heated at constant volume from 35 ⁰C to 75 ⁰C. Determine the change in entropy (in J/K).arrow_forwardA hot-water stream at 80°C enters a mixing chamber with a mass flow rate of 0.5 kg/s where it is mixed with a stream of cold water at 20°C. If it is desired that the mixture leave the chamber at 42°C, determine the mass flow rate of the cold-water stream. Assume all the streams are at a pressure of 250 kPa.arrow_forwardHelium at 200 kPa, 20 ∘C is heated by mixing it with argon at 200 kPa, 500 ∘C in an adiabatic chamber. Helium enters the chamber at 2 kg/s and argon at 0.6 kg/s. The mixture leaves at 200 kPa. (Figure 1) Part A Determine the temperature (T2) at the exit. Express your answer to four significant figures. Part B Determine the rate of entropy generation (S˙gen) due to mixing. Express your answer to three significant figures in kW/K.arrow_forward
- Water at 20 psia and 50F enters a mixing chamber at a rate of 300 lbm/min where it is mixed steadily with steam entering at 20 psia and 240F. The mixture leaves the chamber at 20 psia and 130F, and heat is lost to the surrounding air at 70F at a rate of 180 Btu/min. Neglecting the changes in kinetic and potential energies, determine the rate of entropy generation during this process.arrow_forwardUsing the steam tables, determine the specific enthalpy, specific volume and specific enthalpy for steam at 2800 KPa and at a temperature of 390 C.c. The steam in the question above is compressed at a constant enthalpy to a pressure of 4700 KPa. What is the final temperature, specific enthalpy, specific volume and specific entropy of the steam in this new state?d. What is the change in specific enthalpy, specific volume, temperature and specificentropy?arrow_forwardLiquid methane is commonly used in various cryogenic applications. The critical temperature of methane is 191 K (or –82°C), and thus methane must be maintained below 191 K to keep it in liquid phase. The properties of liquid methane at various temperatures and pressures are given in Table 7–1. Determine the entropy change of liquid methane as it undergoes a process from 110 K and 1 MPa to 120 K and 5 MPa using tabulated properties. What is the error involved in the latter case?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





