
Concept explainers
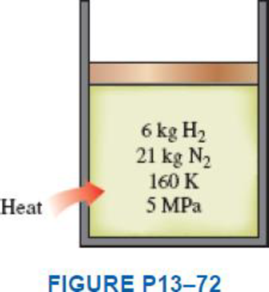
A piston–cylinder device contains 6 kg of H2 and 21 kg of N2 at 160 K and 5 MPa. Heat is now transferred to the device, and the mixture expands at constant pressure until the temperature rises to 200 K. Determine the heat transfer during this process by treating the mixture (a) as an ideal gas and (b) as a nonideal gas and using Amagat’s law.
a)
The heat transfer during the process by treating as an ideal gas.
Answer to Problem 72P
The heat transfer during the process as an ideal gas is
Explanation of Solution
Write a closed system energy balance for the gas mixture.
Here, input energy is
Write the expression to obtain the mole number of
Here, molar mass of
Write the expression to obtain the mole number of
Conclusion:
Refer Table A-1, “Molar mass, gas constant, and critical point properties”, obtain the molar masses of
Substitute
Substitute
From the Table of ideal gas for
Substitute
Thus, the heat transfer during the process as an ideal gas is
b)
The heat transfer during the process by treating as non-ideal gas.
Answer to Problem 72P
The heat transfer during the process by treating as non-ideal gas is
Explanation of Solution
Write the expression to obtain the initial reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial and final reduced pressure of
Here, critical temperature of
Write the expression to obtain the final reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial reduced temperature of
Here, critical temperature of
Write the expression to obtain the initial and final reduced pressure of
Here, critical temperature of
Write the expression to obtain the final reduced temperature of
Here, critical temperature of
Consider hydrogen as ideal gas
Write the expression for molar enthalpy difference of hydrogen
Write the expression for molar enthalpy difference of nitrogen.
Conclusion:
Substitute 160 K for
Substitute 5 MPa for
Substitute 200 K for
Refer Figure A-30, “Generalized entropy departure chart”, obtain the value of
Substitute 160 K for
Substitute 5 MPa for
Substitute 200 K for
Refer Figure A-30, “Generalized entropy departure chart”, obtain the value of
Substitute
Substitute
Substitute
Thus, the heat transfer during the process by treating as non-ideal gas is
Want to see more full solutions like this?
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
- a. 9.23 kg of water vapor is contained at 150 kPa and 90 percent quality in a suitable enclosure. Calculate the heat in kJ which must be added to produce a saturated vapor.b. 9.96 kg of water vapor are contained at 150 kPa and 90 percent quality in a suitable enclosure. What will the pressure be in MPa at the end of the heating process?Please include the h-s and T-s diagram in the solution, and do not round off intermediate answers only the final answer in three decimal places.arrow_forwardWhat is the percentage of error involved in treating carbon dioxide at 5 MPa and 25°C as an ideal gas?arrow_forwardAn insulated piston–cylinder device initially contains 0.01 m3 of saturated liquid–vapor mixture with a quality of 0.2 at 120°C. Now some ice at 0°C is added to the cylinder. If the cylinder contains saturated liquid at 120°C when thermal equilibrium is established, determine the amount of ice added. The melting temperature and the heat of fusion of ice at atmospheric pressure are 0°C and 333.7 kJ/kg, respectivelyarrow_forward
- A 2.712-kg steam-water mixture at 1.0 MPa is contained in an inflexible tank. Heat is added until the pressure rises to 3.5 MPa and the temperature to 400°C. Determine the heat added in kJ.Only the final answer must be in 3 decimal points.arrow_forwardA mixture of ideal gases has a specific heat ratio of k = 1.35 and an apparent molecular weight of M = 44 kg/kmol. Determine the work, in kJ/kg, required to compress this mixture isentropically in a closed system from 100 kPa and 35°C to 700 kPa. The universal gas constant is Ru = 8.314 kJ/kmol·K. The work required to compress this mixture is _______kJ/kg.arrow_forwardIf 10 kg of ice at 0C is added to 2 kg of steam at 100C, the temperature of resulting mixture isarrow_forward
- A tank contains 20 kg of saturated liquid water at 75°C. What is the totalinternal energy of the water in kJ? What is the total enthalpy of the water in kJ?arrow_forwardA 240-m3 rigid tank is filled with a saturated liquid– vapor mixture of water at 200 kPa. If 25 percent of the mass is liquid and 75 percent of the mass is vapor, the total mass in the tank is (a) 240 kg (b) 265 kg (c) 307 kg (d) 361 kg (e) 450 kgarrow_forwardA constant-pressure R-134a vapor separation unit separates the liquid and vapor portions of a saturated mixture into two separate outlet streams. Determine the flow power needed to pass 6 L/s of R-134a at 320 kPa and 55 percent quality through this unit. What is the mass flow rate, in kg/s, of the two outlet streams?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





