
ORGANIC CHEMISTRY LL BUNDLE
4th Edition
ISBN: 9781119761112
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.5, Problem 6ATS
Interpretation Introduction
Interpretation: The reagents used to prepare compound 1 via a
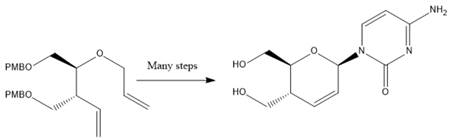
Compound 1
Concept Introduction: In Williamson Ether Synthesis, ether is formed from deprotonated alcohol and an organohalide. The reaction takes place between a primary

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show what reagents you would use to synthesize this ether by each of the following methods, show mechanism for method A and C.
A. Acid-catalyzed ether formation from alcohols
B. Alkoxymercuration-demercuration (do not show mechanism)
C. Williamson ether synthesis
What is the role of phosphoric acid in the synthesis of cyclohexene?
it is an antioxidant that prevents free radical side reactions
it is a safe, non-toxic solvent
it lowers the boiling point of the reaction mixture (a colligative property of adding
phosphoric acid to water)
it protonates the hydroxyl of cyclohexanol to make it a better leaving group
Please don't provide handwritten solution ...
Chapter 13 Solutions
ORGANIC CHEMISTRY LL BUNDLE
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardUsing your reaction roadmap as a guide, show how to convert cyclohexane into hexanedial. Show all reagents and all molecules synthesized along the way.arrow_forwardAccount for the fact that hydroperoxidation of ethers is regioselective (i.e., reaction occurs preferentially at a carbon adjacent to the ether oxygen).arrow_forward
- Begin by classifying the carbon atoms on either side of the oxygen atom. Propose the types of alcohol and halide which can be used for Williamson ether synthesis.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward5. Synthesize the following compounds by using cyclohexanone and propene as your only sources of carbon. Any other needed reagents are available.arrow_forward
- hello! i badly need your help in this. looking forward to your reply asap. thanks!arrow_forwardConvert Compound A to Compound B by coming up with a route for this. Design a route to take A to B be sure to outline the reagents for each step. Use reactions only from organic chemistry 1arrow_forwardConsider the synthesis of the ether below. Provide an appropriate alkyl halide and alkoxide anion to synthesize the compound via a Williamson ether synthesis. Alkyl Halide + Alkoxide Anion H3C- O CH3 Alkyl halide Alkoxide anionarrow_forward
- Terreic acid, shown below, is a naturally occurring antibiotic metabolite of the fungus Aspergillus terreus. Terreic acid hinders bacterial growth by covalently binding to (and thereby deactivating) the biosynthetic enzyme MurA, which is responsible for synthesizing the bacterial cell wall. In aqueous environments, terreic acid tautomerizes to a more stable enol form. Draw the two most stable enol tautomers of terric acid. Circle the tautomer above that you would expect to predominate inside a cellular environment.arrow_forwarda) b) 1. Provide the necessary reagents to carry out the following transformations. 왜 H H OMearrow_forwardProvide the appropriate reagent(s) for the following transformation. dosarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY