
Concept explainers
(a)
Interpretation: The major product in the reaction needs to be identified.
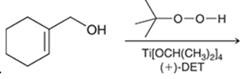
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(b)
Interpretation: The major product in the reaction needs to be identified.
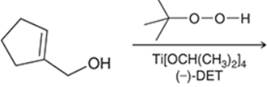
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(c)
Interpretation; The major product in the reaction needs to be identified.
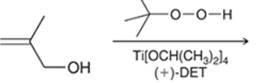
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(d)
Interpretation: The major product in the reaction needs to be identified.
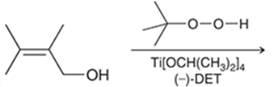
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
ORGANIC CHEMISTRY LL BUNDLE
- Predict all possible products for the following reactions: please clearly show MAJOR product, MINOR product, and TRACE product (if it is possible theoretically but not expected to be observed). If no products are expected, please say NO REACTION: ? O₂N A NO₂ NO₂ HNO3 NO₂ H₂SO4 B NO₂ NO₂ O₂N C NO₂ D NO REACTIONarrow_forwardPredict the product(s) and provide the mechanism for each reaction below.arrow_forwardIdentify the major product in each of the following reactions:arrow_forward
- Predict the major product of the following reaction and draw it in the space provided. 1) NAOMe Br 2) Meo Ome Br 3) NaOt-Bu 4) HCI, H₂O, heatarrow_forwardPredict the major organic product of each of the following reactions or provide the reagent needed to complete each transformation.arrow_forwardPredict the major product for each of the following reactions. H₂C Draw Your Solution e Textbook and Media H PPh3 (EtO) P 1:0 OEt ? →?arrow_forward
- Predict the products for each of the following reactions and provide an explanation for the stereochemistry of the product. NC !! -CNarrow_forwardprovide appropriate reagents or product for the following example if you can show work I'd appreciate it!!! OMe OMearrow_forwardPredict the major product for the following reaction sequence. CI 1) LIAI(OR) H 2) EtMgBr 3) H₂O+ ?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

