
Concept explainers
Propose structures for compounds that fit the following 1H NMR data:
(a) C4H6Cl2
2.18 δ (3 H, singlet)
4.16 δ (2 H, doublet, J=7 Hz)
5.71 δ (1 H, triplet, J=7 Hz)
(b) C10H14
1.30 δ (9 H, singlet)
7.30 δ (5 H, singlet)
(c) C4H7BrO
2.11 δ (3 H, singlet)
3.52 δ (2 H, triplet, J=6 Hz)
4.40 δ (2 H, triplet, J=6 Hz)
(d) C9H11Br
2.15 δ (2 H, quintet, J=7 Hz)
2.75 δ (2 H, triplet, J=7 Hz)
3.38 δ (2 H, triplet, J=7 Hz)
7.22 δ (5 H, singlet)
a)

Interpretation:
The proposed structure of the compound to be identified for the given 1HNMR spectrum.
Concept introduction:
HDI calculation:
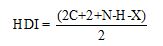
Where
C represent number of carbons.
N represent number of nitrogens.
H represent number of hydrogens.
X represent number of halogens.
Chemical shift: The frequency of the proton signal in the spectrum with reference to the standard compound which may be TMS(Tetramethylsilane) shows signal at 0 ppm(parts per million).
Multiplicity: The number of peaks on the each signal in NMR spectrum is defined as multiplicity; the multiplicity of each signal indicates the neighboring protons. It is generated by coupling of the subjected protons with the neighboring protons (both subjected and neighbor protons are to be chemically not equivalent) separated by either two or three sigma bonds.
Rule: Multiplicity of each signal is calculated using (n+1) rule only when the neighboring protons are chemically equivalent to each other.
(n+1)
where
n indicates number of neighboring protons
Integration value (I): The integration value at the bottom of the 1HNMR spectrum represents the number of protons giving rise to the signal.
To find:
The structure of the compound to be identified for the given molecular formula and 1HNMR spectrum.
Answer to Problem 55GP
Explanation of Solution
Calculate HDI value:
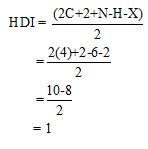
The HDI calculation confirms the presence of an aliphatic ring and double bond
Adjust the relative integration with the number of protons from the molecular formula.
The total number of protons in the molecular formula C10H14 is 6.
Interpret the given information.
Given information:
Three signals with multiplicity and integration values.
2.18ppm(3H, singlet)
4.16ppm(2H, doublet, J=7HZ)
5.71ppm(1H , triplet, J=7HZ)
The HDI value confirms the compound has either a ring or a double bond (one level of unsaturation). The total integration value (3+2+1=6 protons) is also an exact value with the protons of the molecular formula.
A signal at 2.18ppm with integration of 3H’s represents methyl groups which are chemically equivalent having one neighboring proton indicates the characteristic pattern of isopropyl group.
A signal with integration of 2H’s represent a methylene group appears at 4.16ppm rather 5.17ppm, consistent with the value of protons which present at alpha position to vinyl group (C=O) and accounts for the one degree of unsaturation.
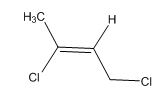
The overall predicted structure is:

The methyl groups can be interchanged via no reflectional symmetry and the compound gives rise to totally three signals in spectrum.
The structure of the compound is identified using the details of spectrum and DHI calculation.
b)
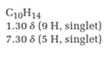
Interpretation:
The proposed structure of the compound to be identified for the given 1HNMR spectrum.
Concept introduction:
HDI calculation:
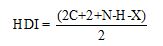
Where
C represent number of carbons.
N represent number of nitrogens.
H represent number of hydrogens.
X represent number of halogens.
Chemical shift: The frequency of the proton signal in the spectrum with reference to the standard compound which may be TMS(Tetramethylsilane) shows signal at 0 ppm(parts per million).
Multiplicity: The number of peaks on the each signal in NMR spectrum is defined as multiplicity; the multiplicity of each signal indicates the neighboring protons. It is generated by coupling of the subjected protons with the neighboring protons (both subjected and neighbor protons are to be chemically not equivalent) separated by either two or three sigma bonds.
Rule: Multiplicity of each signal is calculated using (n+1) rule only when the neighboring protons are chemically equivalent to each other.
(n+1)
where
n indicates number of neighboring protons
Integration value (I): The integration value at the bottom of the 1HNMR spectrum represents the number of protons giving rise to the signal.
To find:
The structure of the compound to be identified for the given molecular formula and 1HNMR spectrum.
Answer to Problem 55GP
Explanation of Solution
Calculate HDI value:
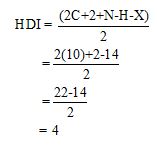
The HDI calculation confirms the presence of an aliphatic ring and double bond
Adjust the relative integration with the number of protons from the molecular formula.
The total number of protons in the molecular formula C10H14 is 14.
Interpret the given information.
Given information:
Three signals with multiplicity and integration values.
1.30ppm(9H, singlet)
7.30ppm(5H, singlet)
The HDI value confirms the compound has either a ring or a double bond (four level of unsaturation). The total integration value (9+5=14 protons) is also an exact value with the protons of the molecular formula.
A signal at 1.30ppm with integration of 9H’s represents methyl groups which are chemically equivalent having one neighboring proton indicates the characteristic pattern of isopropyl group.
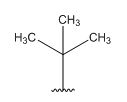
A signal with integration of 5H’s represent a benzene appears at 7.30ppm which present at aromatic group and accounts for the four degree of unsaturation.
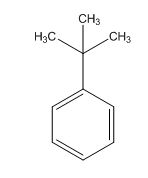
The overall predicted structure is:

The methyl groups can be interchanged via reflectional symmetry and the compound gives rise to totally two signals in 1HNMR spectrum.
The structure of the compound is identified using the details of 1HNMR spectrum and DHI calculation.
c)

Interpretation:
The proposed structure of the compound to be identified for the given 1HNMR spectrum.
Concept introduction:
HDI calculation:
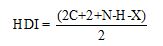
Where
C represent number of carbons.
N represent number of nitrogens.
H represent number of hydrogens.
X represent number of halogens.
Chemical shift: The frequency of the proton signal in the spectrum with reference to the standard compound which may be TMS(Tetramethylsilane) shows signal at 0 ppm(parts per million).
Multiplicity: The number of peaks on the each signal in NMR spectrum is defined as multiplicity; the multiplicity of each signal indicates the neighboring protons. It is generated by coupling of the subjected protons with the neighboring protons (both subjected and neighbor protons are to be chemically not equivalent) separated by either two or three sigma bonds.
Rule: Multiplicity of each signal is calculated using (n+1) rule only when the neighboring protons are chemically equivalent to each other.
(n+1)
where
n indicates number of neighboring protons
Integration value (I): The integration value at the bottom of the spectrum represents the number of protons giving rise to the signal.
To find:
The structure of the compound to be identified for the given molecular formula and 1HNMR spectrum.
Answer to Problem 55GP
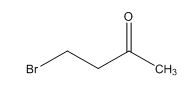
Explanation of Solution
Calculate HDI value:
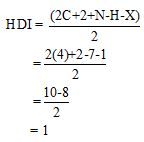
The HDI calculation confirms the presence of an aliphatic ring and double bond
Adjust the relative integration with the number of protons from the molecular formula.
The total number of protons in the molecular formula C4H7Bro is 7.
Interpret the given information.
Given information:
Three signals with multiplicity and integration values.
2.11ppm(3H, singlet)
3.52ppm(2H, triplet, J=6HZ)
4.40ppm(2H, triplet, J=6HZ)
The HDI value confirms the compound has either a ring or a double bond (one level of unsaturation). The total integration value (3+2+2=7 protons) is also an exact value with the protons of the molecular formula.
A signal at 2.11ppm with integration of 3H’s represents methyl groups which are chemically equivalent having one neighboring proton indicates the characteristic pattern of isopropyl group.

A two signal with integration of 2H’s represent a methylene group appears at 3.52ppm rather 4.40ppm, consistent with the value of protons which present at alpha position to carbonyl group(C=O) and accounts for the one degree of unsaturation.
The overall predicted structure is:

The methyl groups can be interchanged via no reflectional symmetry and the compound gives rise to totally three signals in spectrum.
The structure of the compound is identified using the details of 1HNMR spectrum and DHI calculation.
d)
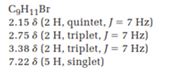
Interpretation:
The proposed structure of the compound to be identified for the given 1HNMR spectrum.
Concept introduction:
HDI calculation:
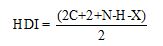
Where
C represent number of carbons.
N represent number of nitrogens.
H represent number of hydrogens.
X represent number of halogens.
Chemical shift: The frequency of the proton signal in the spectrum with reference to the standard compound which may be TMS(Tetramethylsilane) shows signal at 0 ppm(parts per million).
Multiplicity: The number of peaks on the each signal in NMR spectrum is defined as multiplicity; the multiplicity of each signal indicates the neighboring protons. It is generated by coupling of the subjected protons with the neighboring protons (both subjected and neighbor protons are to be chemically not equivalent) separated by either two or three sigma bonds.
Rule: Multiplicity of each signal is calculated using (n+1) rule only when the neighboring protons are chemically equivalent to each other.
(n+1)
where
n indicates number of neighboring protons
Integration value (I): The integration value at the bottom of the spectrum represents the number of protons giving rise to the signal.
To find:
The structure of the compound to be identified for the given molecular formula and 1HNMR spectrum.
Answer to Problem 55GP
Explanation of Solution
Calculate HDI value:
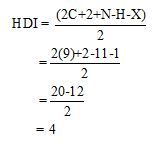
The HDI calculation confirms the presence of an aliphatic ring and double bond
Adjust the relative integration with the number of protons from the molecular formula.
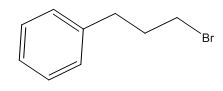
The total number of protons in the molecular formula C9H11Br is 11.
Interpret the given information.
Given information:
Three signals with multiplicity and integration values.
2.15ppm(2H, quintet, J=7HZ)
2.75ppm(2H, triplet, J=7HZ)
3.38ppm(2H, triplet, J=7HZ)
7.22ppm(5H, singlet)
The HDI value confirms the compound has either a ring or a double bond (four level of unsaturation). The total integration value (2+2+2+5=11 protons) is also an exact value with the protons of the molecular formula.
A signal at 2.15ppm with integration of 2H’s represents three methyl groups which are chemically equivalent having one neighboring proton indicates the characteristic pattern of alkyl group.
A signal with integration of 2H’s represent benzylic appears at 2.75ppm which present at aromatic group
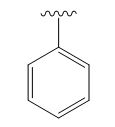
A signal with integration of 5H’s represent benzylic appears at 7.30ppm which present at aromatic group and accounts for the four degree of unsaturation.
The overall predicted structure is:

The methyl groups can be interchanged via reflectional symmetry and the compound gives rise to totally four signals in spectrum.
The structure of the compound is identified using the details of 1HNMR spectrum and DHI calculation.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry
- Propose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardThe 1H-NMR spectrum of compound R, C6H14O, consists of two signals: d 1.1 (doublet) and d 3.6 (septet) in the ratio 6:1. Propose a structural formula for compound R consistent with this informationarrow_forward
- The 1H-NMR spectrum of compound B,C7H14O , consists of the following signals: δ 0.9 (t, 6H), 1.6 (sextet, 4H), and 2.4 (t, 4H). Draw the structural formula of compound B.arrow_forwardA compound (C7H14O) has a strong peak in its IR spectrum at 1710 cm–1. Its 1H NMR spectrum consists of three singlets in the ratio 9:3:2 at δ 1.0, 2.1, and 2.3, respectively. Identify the compound.arrow_forwardProvide a structure for the compound with molecular formula C5H10O and with the following spectroscopic data.IR: 1720 cm−11H NMR: 0.9δ (triplet, I=3H), 1.7δ (sextet, I=2H), 2.1δ (singlet, I=3H), 2.4δ (triplet, I=2H)arrow_forward
- Compound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound. Please show work.arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forwardPropose a structure for a compound of molecular formula C7H14O2 with an IR absorption at 1740 cm−1 and the following 1H NMR data: Absorption ppm Relative area singlet 1.2 9 triplet 1.3 3 quartet 4.1 2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

