
Each of the following names is wrong. Give the structure and correct name of
each compound.
a
b
c.
d.
(a)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

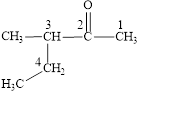
Figure 1
The longest carbon chain contains five carbon atoms. One methyl substituent is attached to the third carbon atom. The functional group present in the given compound is ketone
The structural formula is shown in Figure 1.
The correct name of the compound is
(b)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

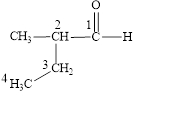
Figure 2
The longest carbon chain contains four carbon atoms. One methyl substituent is attached to the second carbon atom. The functional group present in the given compound is aldehyde
The structural formula is shown in Figure 2.
The correct name of the compound is
(c)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

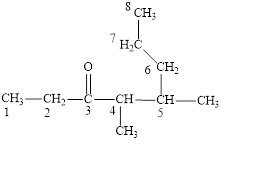
Figure 3
The longest carbon chain contains eight carbon atoms. One methyl substituent is attached to the fourth carbon atom and the other methyl group is attached to the fifth carbon atom. The functional group present in the given compound is ketone
The structural formula is shown in Figure 3.
The correct name of the compound is
(d)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.11E
The structural formula of
The correct name is
Explanation of Solution
The given name is

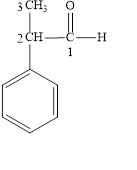
Figure 4
The longest carbon chain contains three carbon atoms. One phenyl substituent is attached to the second carbon atom. The functional group present in the given compound is aldehyde
The structural formula is shown in Figure 4.
The correct name of the compound is
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Select the functional group with the lowest oxidation state. a. carboxylic acid b. nitrile c. alkene d. ketonearrow_forwardA student was given the structural formulas of several compounds and was asked to give them systematic names. How many did she name correctly? Correct those that are misnamed. a. 4-ethyl-2-pentyne b. 1-bromo-4-heptyne c. 2-methyl-3-hexyne d. 3-pentynearrow_forwardTrans-3-methylhex-2-ene-2-anol 2-methylhexanoic acid 1-chloro-3-fluorobenzene N-butyl-N-ethyl-2-pentanamine Complete their structural diagram and class/ family for each.arrow_forward
- Please mark the false statement below about reactions. A. The main product formed as a result of the reaction of 2-bomo-2-methyl propane with NaOH is 2-methyl-2-hydroxy propane. B. The main product formed as a result of the reaction of 2-bomo-2-methyl propane with NaOH is 2-methyl-propene. C. The main product formed as a result of the reaction of 2-bomo-2-methyl propane with NaOH is 2-methyl-propene D. The reaction of 1-bromo-2,3-dimethyl butane in zinc acetic acid medium results in a saturated hydrocarbon. E. The reaction of 2,3-dibromo-2,3-dimethyl butane in zinc acetic acid is an unsaturated hydrocarbon.arrow_forwardWhat is the structure of molecule A? ( Fenol means phenol) a) Cyclohexylamine b) Aniline c) Benzene d) Anisole e) aminohexanearrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecynearrow_forward
- Fill in the blanks; ______ has a higher boiling point than _________? a. Carbon dioxide; 1-butanamine b. Butane; 2-butanol c. None of these are correct d. 1-methoxypropane; 2-heptene e. Cyclohexane; 1-propanolarrow_forwardConsider a compound with a formula: C5H10O (5 carbons, 10 hydrogens, one oxygen). A correct IUPAC name for one of ketone isomers of this compound is a 3-Methybutanal b 4-Methylbutanone c 3-Methylbutanone d None of the abovearrow_forwardWhat is the product of pentene and water under acidic conditions? Group of answer choices pentanol 2-pentanone pentanoic acid pentanalarrow_forward
- Complete the following reactions by filling in the necessary reacents. ? 3,4-diphenyl-1-butene 3, 4- diphenyl butanoic acidarrow_forward2-methylhexanoic acid can be synthesized by oxidation of which of the following compounds? A) 2-methylhexane B) 2-methyl-1-hexanol C) 2-methyl-1-hexene D) 2-methyl-1-hexyne E) 2-methyl-2-hexanonearrow_forwardDraw the structure of the 3-phenylpentane.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

