
Concept explainers
(a)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming thioalcohols that contain single thiol group:
- • Longest carbon chain has to be identified that contains thiol group also. The chain name is obtained by adding the suffix “-thiol”. If the compound contains an unsaturated bond, then the respective name has to be changed with regard to
alkane . - • The numbering has to be given so that the thiol group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the thiol group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
IUPAC rules for naming thioalcohols that contain more than one thiol group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of thiol groups that is present.
(a)
Answer to Problem 14.140EP
IUPAC name for the given compound is 2-hexanethiol.
Explanation of Solution
Given structure of compound is shown below,
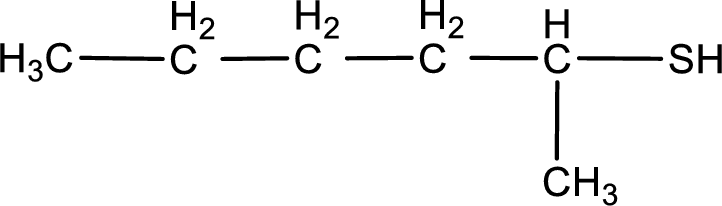
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is six carbon chain. Hence, the parent alkane is hexane.
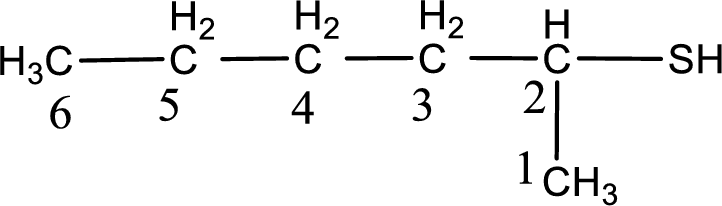
The suffix -thiol is added to the parent alkane name. This gives the name as hexanethiol.
Next step is to number the carbon atoms so that the thiol functional group gets the least numbering. In this case, it is in second carbon atom. Therefore, the IUPAC name of the given compound is 2-hexanethiol.
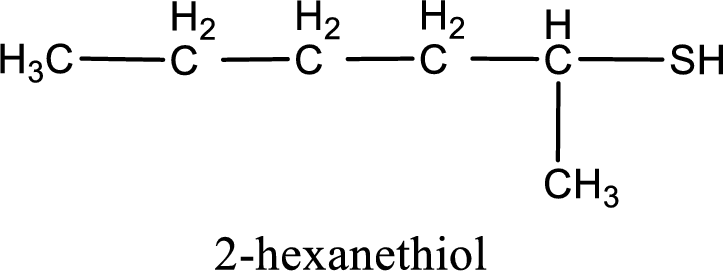
IUPAC name for the given compound is assigned.
(b)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming alcohols that contain single hydroxyl group:
- • Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”. If the compound contains a unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the hydroxyl group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- • Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
- • If the compound contains bulky groups on same side of the double bond, then it is a cis isomer and if the bulkyl groups are present on opposite side of the double bond, then it is a trans isomer.
- • In case of cycloalkane compounds, if the substitutions are present on same side of the ring of carbon atoms, it is a cis isomer. If the substitutions are present above and below the ring, then it is a trans isomer.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
(b)
Answer to Problem 14.140EP
IUPAC name for the given compound is 2-hexanol.
Explanation of Solution
Given structure of compound is shown below,
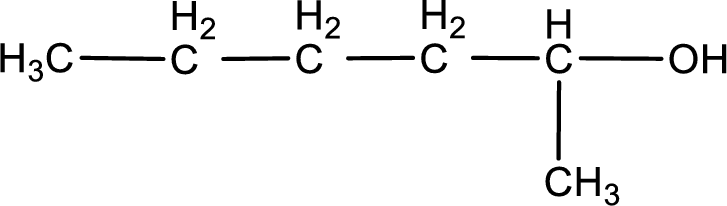
The longest continuous carbon chain with the hydroxyl group is found to be six carbon chain. Therefore, the parent alkane is hexane. As a hydroxyl group is present the name of the alcohol can be given as hexanol.
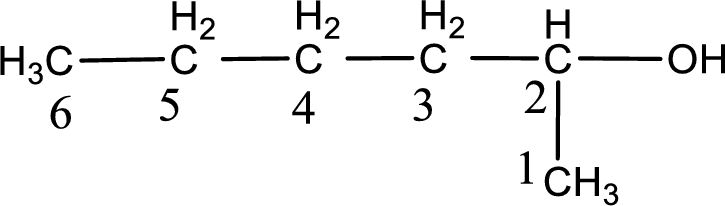
The numbering has to be given so that the hydroxyl group gets the least numbering. In this case, the hydroxyl group is present in the second carbon atom. Therefore, the IUPAC name can be given as 2-hexanol.
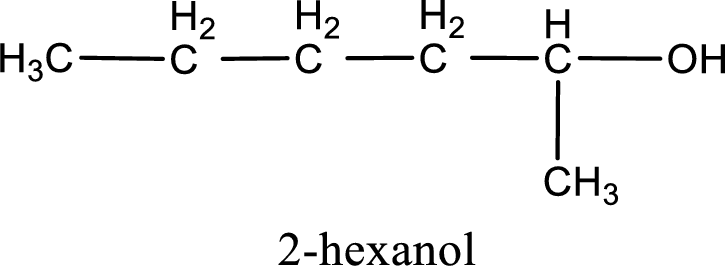
IUPAC name for the given compound is assigned.
(c)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming alcohols that contain single hydroxyl group:
- • Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”. If the compound contains a unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the hydroxyl group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- • Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
- • If the compound contains bulky groups on same side of the double bond, then it is a cis isomer and if the bulkyl groups are present on opposite side of the double bond, then it is a trans isomer.
- • In case of cycloalkane compounds, if the substitutions are present on same side of the ring of carbon atoms, it is a cis isomer. If the substitutions are present above and below the ring, then it is a trans isomer.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
(c)
Answer to Problem 14.140EP
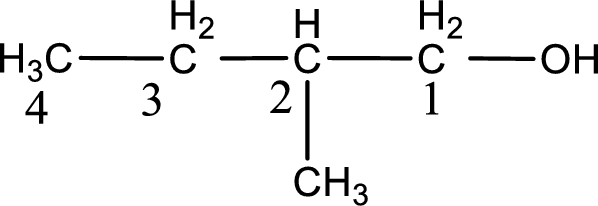
IUPAC name for the given compound is 2-methyl-1-butanol.
Explanation of Solution
Given structure of compound is shown below,
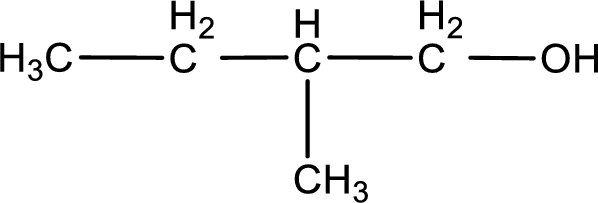
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is four carbon chain. Hence, the parent alkane is butane.
The suffix -ol is added to the parent alkane name by replacing the suffix “–e”. This gives the name as butanol.
Next step is to number the carbon atoms so that the hydroxyl functional group gets the least numbering. In this case, it is in first carbon atom. Methyl group is present as substituent in the second carbon atom. Therefore, the IUPAC name of the given compound is 2-methyl-1-butanol.
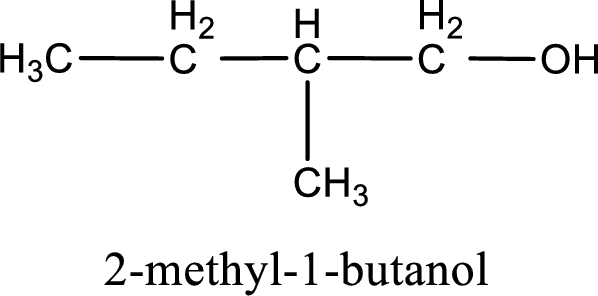
IUPAC name for the given compound is assigned.
(d)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
IUPAC rules for naming thioalcohols that contain single thiol group:
- • Longest carbon chain has to be identified that contains thiol group also. The chain name is obtained by adding the suffix “-thiol”. If the compound contains an unsaturated bond, then the respective name has to be changed with regard to alkane.
- • The numbering has to be given so that the thiol group gets the least numbering.
- • Name and location of any other substituent present in the chain has to be identified.
- • If in a ring the thiol group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
IUPAC rules for naming thioalcohols that contain more than one thiol group:
- • The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of thiol groups that is present.
(d)
Answer to Problem 14.140EP
IUPAC name for the given compound is 2-methyl-1-butanethiol.
Explanation of Solution
Given structure of compound is shown below,
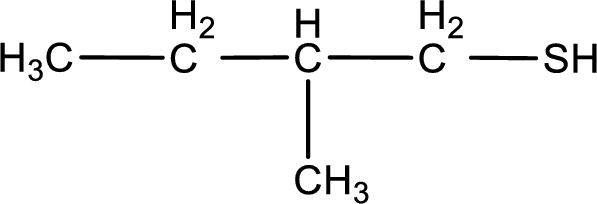
First step is to identify the longest continuous carbon chain. In the given structure, it is found that the longest carbon chain is four carbon chain. Hence, the parent alkane is butane.
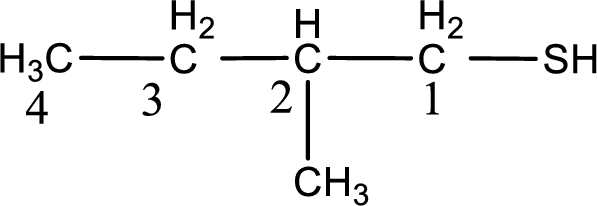
The suffix -thiol is added to the parent alkane name. This gives the name as butanethiol.
Next step is to number the carbon atoms so that the thiol functional group gets the least numbering. In this case, it is in first carbon atom. Methyl group is present as substituent in the second carbon atom. Therefore, the IUPAC name of the given compound is 2-methyl-1-butanethiol.
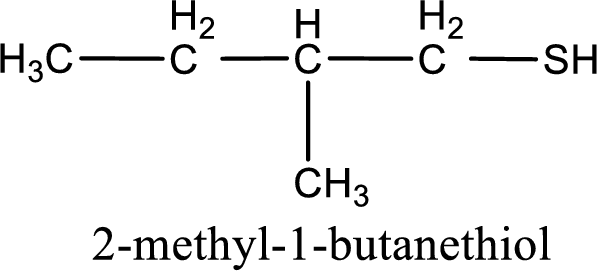
IUPAC name for the given compound is assigned.
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, and Biological Chemistry
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


