
Concept explainers
Interpretation:
A VB picture of the
Concept introduction:
Some chemical and structural features of molecules are unaccounted for by VB theory, most notably electron delocalization, or resonance, over three or more nuclei. The VB theory picture of a species that has resonance is inaccurate because it represents a single resonance structure, not the hybrid.
Answer to Problem 14.1P
The VB picture of the
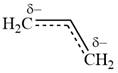
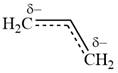
The structure of the resonance hybrid of allyl anion is
Explanation of Solution
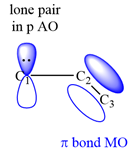
All three carbon atoms in the allyl anion are
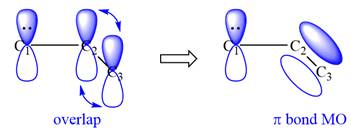
This corresponds to a double bond between C2 and C3, with a
The VB theory picture of a given species represents a single resonance structure and not the hybrid.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Consider the incomplete orbital representation of O2 , below right. a. Identify which lobes are hybrid orbitals (identify the type) and which lobes arep orbitals. b. Use dotted lines to show any bonds. c. Use up or down arrows to show electron occupation of each hybrid orbital or bond.arrow_forwardDraw a molecule that contains at least one sp-hybridized carbon and at least 1 sp2- hybridized carbon. Label those two carbons (one sp- and one sp2-hybridized.)arrow_forwardThe structure of urea is shown below (attached image). Make sure to add any missing lone pairs. In urea there are ______ sp3 hybridized atoms and _____ sp2 hybridized atoms. Remember, hydrogens can't hybridize their s orbital, so it is best to not to count them.arrow_forward
- Draw a bond-line structure that best matches the given 3D representation?arrow_forwardWhat is the molecular formula?For each heteroatom (not C or H), indicate the hybridization. Also note whether each lone pair is localized (on the atom) or delocalized (through resonance).arrow_forwardThe problem is asking how many Sp3, Sp2, and Sp atoms there are and the answer key says 16 Sp3, 8 Sp2 and 2 Sp but I'm not even counting 26 carbons on this molecule so I'm a little confused.arrow_forward
- Determine the correct hybridizaton (from left to right) about each interior atom in CH≡CCH2ClCH≡CCH2Cl.arrow_forwardDraw DMAP in bond line format with all lone pairs, as needed. What is the hybridzation of the two different N atoms?arrow_forwardPoint out the hybridization situation (sp3, sp2, sp, or not hybridized) of the highlighted atoms below.: note that you are responsible for adding proper number of lone pairs wherever necessary. C(highlighted) on the left is __________ hybridized; so the bond angle CCC (around the same carbon) is O(highlighted) on the right is ___________ hybridized; so the bond angle COC (around the oxygen) is _______ N(highlighted) at the top-left corner is __________________ hybridized; so the bond angle NCC is __________ . N(highlighted) on the right is __________________ hybridized; so the bond angle CNC is __________ .arrow_forward
- Identify all of the carbon atoms that are sp2-hybridized in the following molecule:arrow_forwardA student argues the the two nitrogen’s in the compound below are sp2 hybridized, but atom a is still more basic than atom b. Is the student correct? Explain.arrow_forwardLet us construct the molecular orbital diagram of ethylene (in pieces). a. First, construct the MO diagram of linear carbene (CH2). Draw pictures of all 6 orbitals b. Now bend the carbene to a bond angle of about 120°. How does this change your MO diagram? Draw pictures of all 6 orbitals. c. Now bring two of these carbene molecules together to make ethylene. Draw pictures of all 12 orbitals.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
