
Concept explainers
(a)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
In the given compound, carbon atom of labeled proton
The signal of proton
(b)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
In the given compound, carbon atom of labeled proton
The signal of proton
(c)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
In the given compound, carbon atom of labeled proton
The signal of proton
(d)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
In the given compound, carbon atom of labeled proton
The signal of proton
(e)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
In the given compound, carbon atom of labeled proton
The signal of proton
(f)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
The splitting pattern shown by each labeled proton is show in Figure 1.
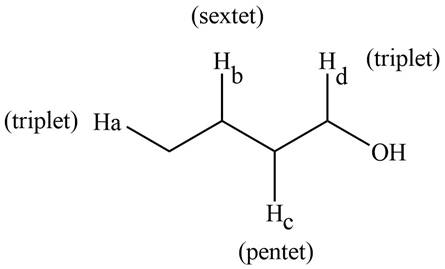
Figure 1
The signal of proton
(g)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
The splitting pattern shown by each labeled proton is show in Figure 2.
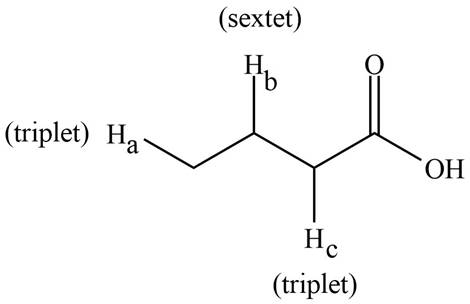
Figure 2
The signal of proton
(h)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
The splitting pattern shown by each labeled proton is show in Figure 3.
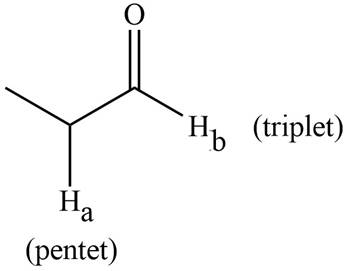
Figure 3
The signal of proton
(i)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
The splitting pattern shown by each labeled proton is show in Figure 4.
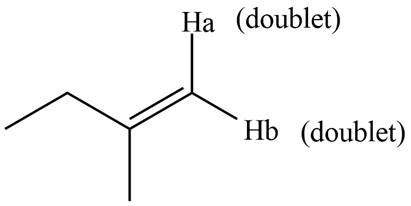
Figure 4
The signal of proton
(j)
Interpretation: The number of peaks into which each single of labeled proton will split is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 14.42P
The signal of proton
Explanation of Solution
In NMR spectroscopy, splitting of signal takes place according to number of protons present on neighboring carbon atom. The number of peaks into which a signal will split is
The splitting pattern shown by each labeled proton is show in Figure 5.
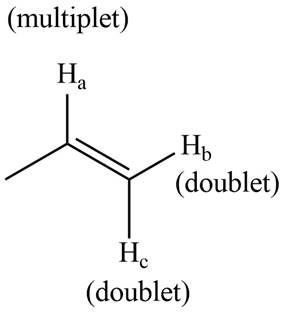
Figure 5
The signal of proton
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- match the molecule that corresponds to the mass spectrumarrow_forwardFor the following mass spectra determine: presence or absence of Cl and Br, molecular weight, of it is consistent with an odd number or zero/even number of N atoms, parent peak, base peak.arrow_forwardhow to draw initial structure that matches signals. assigning spectra.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
