
Identify each of the following compounds as an
a.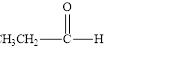 d.
d.
b.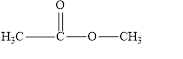 e.
e.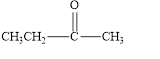
c.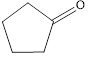 f.
f.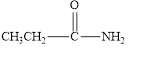
(a)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to minimum one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is an aldehyde.
Explanation of Solution
The given compound is shown below.
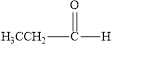
Figure 1
The given compound contains carbonyl group that is connected to one carbon atom and one hydrogen atom. Hence, it is an aldehyde.
The given compound is an aldehyde.
(b)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to minimum one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is neither aldehyde nor ketone.
Explanation of Solution
The given compound is shown below.
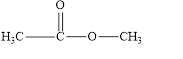
Figure 2
In the given compound, the carbonyl group is not attached with two carbon atoms. Therefore, it is not a ketone. Also, the carbonyl group is not attached with at least one hydrogen atom. Thus, it is not an aldehyde. Hence, it is neither aldehyde nor ketone.
The given compound is neither aldehyde nor ketone.
(c)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to minimum one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is a ketone.
Explanation of Solution
The given compound is shown below.
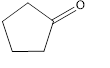
Figure 3
The given compound contains carbonyl group that is connected to two carbon atoms in the ring. Hence, it is a ketone.
The given compound is a ketone.
(d)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to minimum one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is neither aldehyde nor ketone.
Explanation of Solution
The given compound is shown below.
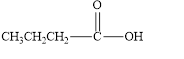
Figure 4
In the given compound, the carbonyl group is not attached with two carbon atoms. Therefore, it is not a ketone. Also, the carbonyl group is not attached with at least one hydrogen atom. Thus, it is not an aldehyde. Hence, it is neither aldehyde nor ketone.
The given compound is neither aldehyde nor ketone.
(e)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to at least one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is a ketone.
Explanation of Solution
The given compound is shown below.
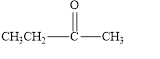
Figure 5
The given compound contains carbonyl group that is connected to two carbon atoms. Hence, it is a ketone.
The given compound is a ketone.
(f)
Interpretation:
The given compound is to be identified as an aldehyde, a ketone, or neither.
Concept introduction:
An aldehyde consists of a carbonyl group that is single bonded to minimum one hydrogen atom, whereas a ketone consists of a carbonyl group that is single bonded to two carbon atoms. The structural formula of an aldehyde is
Answer to Problem 14.4E
The given compound is neither aldehyde nor ketone.
Explanation of Solution
The given compound is shown below.
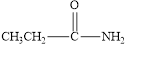
Figure 6
In the given compound, the carbonyl group is not attached with two carbon atoms. Therefore, it is not a ketone. Also, the carbonyl group is not attached with at least one hydrogen atom. Thus, it is not an aldehyde. The carbonyl group is attached to one nitrogen atom and one carbon atom. Hence, it is neither aldehyde nor ketone.
The given compound is neither aldehyde nor ketone.
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Give the structure of an alcohol that could be used to prepare each of the following compounds: a. b. c.arrow_forwardAlkanes are good organic solvents and miscible in non-polar solvent. Why alkanes are insoluble in water?arrow_forwardDraw the condensed formulas for each of the following compounds: (a) dipropyl ether. (b) 2, 2-dimethyl-3-hexanol. (c) 2-ethoxybutanearrow_forward
- Assign the IUPAC name to each of the following ethers. Name the smaller alkyl group as the alkoxy group. a. CH3CH2OCH2CH2CH3 b. c. d.arrow_forward. Give the systematic name for each of the following unsaturated hydrocarbons and substituted unsaturated compounds. a. b. c. d.arrow_forwardExplain why propane boils at 42C, whereas ethanal, which has the same molecular weight, boils at 20C.arrow_forward
- . What is the simplest aromatic alcohol commonly called? What is it mostly used for in the United States?arrow_forwardDraw the structures of the chief product formed when the following alcohols are dehydrated to alkenes: a. b.arrow_forwardExplain the following: a. 1-Hexanol has a higher boiling point than 3-hexanol. b. Diethyl ether has very limited solubility in water, but tetrahydrofuran is completely soluble.arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





