
Concept explainers
(a)
Interpretation:
The IUPAC name of the given compound is to be assigned.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
Answer to Problem 14.5E
The IUPAC name of the given aldehyde is ethanal.
Explanation of Solution
The given compound is shown below.
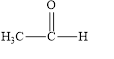
Figure 1
The given compound is aldehyde. The first step in the naming of aldehyde is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -al. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of two
The given aldehyde is ethanal.
(b)
Interpretation:
The IUPAC name of the given compound is to be assigned.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.5E
The IUPAC name of the given aldehyde is
Explanation of Solution
The given compound is shown below.
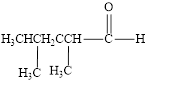
Figure 2
The given compound is aldehyde. The first step in the naming of aldehyde is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -al. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of five
The given aldehyde is
(c)
Interpretation:
The IUPAC name of the given compound is to be assigned.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.5E
The IUPAC name of the given ketone is
Explanation of Solution
The given compound is shown below.
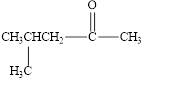
Figure 3
The given compound is ketone. The first step in the naming of ketone is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of five
The given ketone is
(d)
Interpretation:
The IUPAC name of the given compound is to be assigned.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.5E
The IUPAC name of the given aldehyde is
Explanation of Solution
The given compound is shown below.
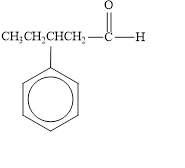
Figure 4
The given compound is aldehyde. The first step in the naming of aldehyde is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -al. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of five
The given aldehyde is
(e)
Interpretation:
The IUPAC name of the given compound is to be assigned.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.5E
The IUPAC name of the given cyclic ketone is
Explanation of Solution
The given compound is shown below.
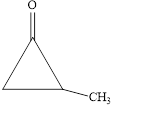
Figure 5
The given compound is cyclic ketone. The first step in the naming of ketone is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of three
The given cyclic ketone is
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Assign the IUPAC name to each of the following ethers. Name the smaller alkyl group as the alkoxy group. a. CH3CH2OCH2CH2CH3 b. c. d.arrow_forwardGive the structure of an alcohol that could be used to prepare each of the following compounds: a. b. c.arrow_forwardDraw the structure of the following compound given the IUPAC name.arrow_forward
- Answer the following questions about A, depicted in the ball-and-stick model.arrow_forwardWhat reagents are used in the esterification of Alcohols and Phenols? a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloridearrow_forwardAssign the IUPAC names to the following aldehydesarrow_forward
- Explain why A is less water soluble than B, even though both compounds have the same functional groups. (See attachment)arrow_forwardWrite the chemical equations for tollen's test reaction for (Use structural formula) a. n-hexane b. cyclohexene c. benzene d. toluenearrow_forwardIn the IUPAC name for the following ketone, it is not common to use a number for the position of the carbonyl group. Why not?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



