
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.68E
Which of the following would be classified as a
a.
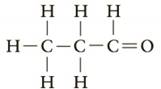
b.
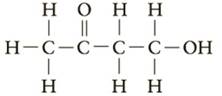
c.
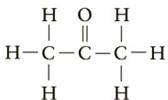
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why A is less water soluble than B, even though both compounds have the same functional groups. (See attachment)
Refer to the given structure and answer the following questions:
What's the unknown ketone and alcohol?
Chapter 14 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 14 - Prob. 14.1ECh. 14 - Prob. 14.2ECh. 14 - Identify each of the following compounds as an...Ch. 14 - Identify each of the following compounds as an...Ch. 14 - Prob. 14.5ECh. 14 - Prob. 14.6ECh. 14 - Prob. 14.7ECh. 14 - Prob. 14.8ECh. 14 - Draw structural formulas and give IUPAC names for...Ch. 14 - Draw structural formulas and give IUPAC names for...
Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Prob. 14.13ECh. 14 - Prob. 14.14ECh. 14 - Prob. 14.15ECh. 14 - Explain why propane boils at 42C, whereas ethanal,...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Prob. 14.19ECh. 14 - Prob. 14.20ECh. 14 - Prob. 14.21ECh. 14 - Prob. 14.22ECh. 14 - Prob. 14.23ECh. 14 - Prob. 14.24ECh. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following structures as a cyclic...Ch. 14 - Label each of the following structures as a...Ch. 14 - What two functional groups react to form the...Ch. 14 - Hemiacetals are sometimes referred to as potential...Ch. 14 - Complete the following statements: a. Oxidation of...Ch. 14 - Prob. 14.32ECh. 14 - Prob. 14.33ECh. 14 - Prob. 14.34ECh. 14 - Prob. 14.35ECh. 14 - Not all aldehyde give a positve Bendicts test....Ch. 14 - A stockroom assistant prepares three bottles, each...Ch. 14 - Glucose, the sugar present within the blood, gives...Ch. 14 - Fructose, present with glucose in honey, reacts...Ch. 14 - Prob. 14.40ECh. 14 - Prob. 14.41ECh. 14 - Complete the following equations. If no reaction...Ch. 14 - Complete the following equations. If no reaction...Ch. 14 - Describe the products that result when hydrogen...Ch. 14 - Prob. 14.45ECh. 14 - Draw structural formulas for the products of the...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - Write equations to show how the following...Ch. 14 - Prob. 14.50ECh. 14 - Identify the most important aldehyde and ketone...Ch. 14 - Using Table 14.3, name an aldehyde or ketone used...Ch. 14 - Prob. 14.53ECh. 14 - CH3COH(O)CH3COOHacetaldehydeaceticacid You need to...Ch. 14 - The addition of water to aldehydes and ketones...Ch. 14 - Prob. 14.56ECh. 14 - Formaldehyde levels above 0.10mg/1000L of ambient...Ch. 14 - In the IUPAC name for the following ketone, it is...Ch. 14 - Why can formaldehyde (CH2O) be prepared in the...Ch. 14 - Other addition reactions of aldehydes occur....Ch. 14 - Prob. 14.61ECh. 14 - Prob. 14.62ECh. 14 - Vanilla flavoring is either extracted from a...Ch. 14 - Prob. 14.64ECh. 14 - The use of acetone in laboratory experiments must...Ch. 14 - Prob. 14.66ECh. 14 - Prob. 14.67ECh. 14 - Which of the following would be classified as a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why propane boils at 42C, whereas ethanal, which has the same molecular weight, boils at 20C.arrow_forwardCyclic esters and amides are called lactones and lactams, respectively. These compounds are classified based on the carbon that is attached to the heteroatom of the ring. Classify the following compounds as a, b, g or d lactones or lactamsarrow_forwardWrite a condensed structural formula for the following compounds: a. b.arrow_forward
- Propane, CH3CH2CH3, is a gas at room temperature, whereas propanol, CH3CH2CH2OH, is a liquid. Why the difference?arrow_forwardHow many mL of a 0.100M NaOH solution would be needed to titrate 0.156g of butanoic acid to a neutral pH endpoint?arrow_forwardIn the IUPAC name for the following ketone, it is not common to use a number for the position of the carbonyl group. Why not?arrow_forward
- Based off of the diagram, please state the # of amide bonds and please state the # of alcohol bonds. Amide Bonds = ? Alcohol Bonds = ?arrow_forwardPhenols are typically _________ acidic than similar alcohols due to ___________ and are __________ in water.arrow_forwardWhich of the following statements about acetone is/are correct?I. Large amount of acetones are produced in the human body.II. Diabetic patients produces larger amounts of acetone.III. In severe diabetes, odor of acetone can be detected on the person's breath.IV. Acetone is partially a by-product in gasoline treatments designed for engine immiscibility to water.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY