
Concept explainers
14-8 Answer true or false.
- The functional group of an alcohol is the —OH (hydroxyl) group.
(h, The solubility of alcohols in water increases with increasing molecular weight.
(a)
Interpretation:
To analyse whether the given statement: The functional group of an alcohol is the −OH (hydroxyl) group, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
The functional group of an alcohol is the −OH (hydroxyl) group. Thus, the statement is true.
Explanation of Solution
The functional group of any alcohol is the —OH group. Therefore, the given statement is true.
(b)
Interpretation:
To analyse whether the given statement: The parent name of an alcohol is the name of the longest carbon chain that contains the −OH group, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
The parent name of an alcohol is the name of the longest carbon chain that contains the −OH group.Thus, the statement is true.
Explanation of Solution
According to IUPAC nomenclature, the parent name of an alcohol is the name of the longest.
carbon chain that contains the —OH group. Therefore, the given statement is true.
(c)
Interpretation:
To analyse whether the given statement: A primary alcohol contains one −OH group, and a tertiary alcohol contains three-OH groups, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
A primary, secondary, and tertiary alcohol contains only one -OH groups but the connectivity of carbon atoms bearing −OH group is different. Thus, the statement is false.
Explanation of Solution
Primary alcohol is alcohol in which the -OH group is linked to carbon that is connected to only one carbon atom. It has like -CH2 OH group in molecule. Secondary alcohol is alcohol in which the -OH group is linked to carbon that is connected to two other carbon atoms. Tertiary alcohol is alcohol in which the -OH group is linked to carbon that is connected to three other carbon atoms. Primary, secondary, and tertiary alcohols have only one -OH group in their molecule but the connectivity of carbon atom bearing -OH group is different. Therefore, the given statement is false.
(d)
Interpretation:
To analyse whether the given statement: In the IUPAC system, the presence of three −OH groups is shown by the ending −triol, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
In the IUPAC system, the presence of three −OH groups is shown by the ending −triol. Thus, the statement is true.
Explanation of Solution
In IUPAC nomenclature, the presence of three -OH groups is shown by the ending triol Therefore, the given statement is true.
(e)
Interpretation:
To analyze whether the given statement: A glycol is a compound that contains two-OH groups. The simplest glycol is ethylene glycol,
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
A glycol is a compound that contains two-OH groups. The simplest glycol is ethylene glycol,
Explanation of Solution
Glycol is a compound that has two -OH groups attached to carbon atoms. Ethyl glycol is the simplest form of glycol. Its structural formula is:
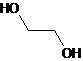
Therefore, the given statement is true.
(f)
Interpretation:
To analyze whether the given statement: Because of the presence of an-OH group, all alcohols are polar compounds, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
Because of the presence of an -OH group, all alcohols are polar compounds. Thus, the statement is true.
Explanation of Solution
Polar molecules have polar covalent bonds connected between the atoms. Methanol is an example of alcohol. Here, the O-C bond and O-H bond are polar covalent bonds. In methanol, there is a difference in electronegativity between the O-C bond (3.5-2.5 = 1.0) and O-H bond (3.5-2.1 = 1.4) Electronegativity is the tendency of an atom to attract electrons towards itself. Due to the presence of the O-H bond, all alcohols are polar molecules.
Therefore, the given statement is true.
(g)
Interpretation:
To analyze whether the given statement: The boiling point of alcohols increases with increasing molecular weight, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
The boiling point of alcohols increases with increasing molecular weight. Thus, the statement is true.
Explanation of Solution
The temperature at which the atmospheric pressure is equal to the vapor pressure is known as boiling point. As the molecular weight increases, more atoms are involved in the non-covalent interaction. Due to this tact, more energy (more temperature) is required to break this non-covalent interactions.Therefore, the given statement is true.
(h)
Interpretation:
To analyze whether the given statement: The solubility of alcohols in water increases with increasing molecular weight, is true or not.
Concept Introduction:
Alcohols are those organic compounds wherein a hydrogen atom of the aliphatic carbon molecule is substituted by a hydroxyl group. Hence an alcohol molecule has two parts, one comprising the alkyl group and the other carrying hydroxyl group.
The boiling point of alcohols is significantly higher than those of alkanes having similar molecular weights. The solubility of alcohol is inversely proportional to the size of the alkyl group. Alcohols reacts with active metals like sodium, potassium and many more to form the corresponding alkoxide.
Answer to Problem 14.8P
The solubility of alcohols in water decreases with increasing molecular weight. Thus, the statement is false.
Explanation of Solution
Alcohols are more soluble in water than hydrocarbons. This is due to the presence of the O-H group in alcohol. The O-H group undergoes hydrogen bonding with a hydrogen atom in the water molecule.
As the molecular weight increases, simultaneously the hydrocarbon chain in alcohol will also increase. Now the alcohol stars behaving more like hydrocarbons. Therefore, the solubility decreases with the increase in molecular weight.
Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
- 13-25 Answer true or false. (a) Phenols and alcohols have in common the presence of an —OH group. Phenols are weak acids and react with strong bases to give water-soluble salts. The pK„ of phenol is smaller than that of acetic acid. Autoxidation converts an R—H group to an R—OH group. A carbon radical has only seven electrons in the valence shell of one of its carbons, and this carbon bears a positive charge. (f, A characteristic of a chain initiation step is conversion of a nonradical to a radical. Autoxidation is a radical-chain reaction. A characteristic of the chain propagation step is reaction of a radical and a molecule to form a new radical and a new molecule. Vitamin E and other natural antioxidants function by interrupting the cycle of chain propagation steps that occurs in autoxidation.arrow_forward14-78 Consider alkenes A, B, and C. each of which has the same molecular formula, C(.H12. Alkenes B and C can each be separated into cis and trans isomers. Upon catalytic reduction using H,, in the presence of a transition metal catalyst (Ni, Pd, or Pt>, alkenes A, B, and C all give hexane as the only product. Acid- catalyzed hydration of alkene C gives one alcohol with the molecular formula CeH14O. Acid catalyzed- hydration of alkene B gives an equal mixture of two alcohols, each with the molecular formula C6H14O. Acid-catalyzed hydration of alkene C gives only a single alcohol with the molecular formula C6H14O. Propose structural formulas for alkenes A, B, and C and the alcohols formed by acid-catalyzed hydration of each, consistent with these experimental results.arrow_forward14-22 Arrange these compounds in order of increasing boiling point. Values in °C are —42, 78, 117, and 198 CH3CH2CH2CH2OH CH3CH2OH HOCH2CH2OH ch3ch2ch3arrow_forward
- 12-50 Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give the indicated alcohol as the major product. More than one alkene may give each alcohol as the major product. 3-Hexanol 1-Methylcyclobutanol 2-Methyl-2-butanol 2-Propanolarrow_forward14-48 Explain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward14-9 What is the difference in structure between a primary, a secondary, and a tertiary alcohol?arrow_forward
- 14-33 Write equations for the reaction of 1-butanol, a primary alcohol, with these reagents. H2SO4, heat K2Cr2O7, H2SO4arrow_forward14-17 Explain in terms of noncovalent interactions why the low-molecular-weight alcohols are soluble in water but the low-molecular-weight alkanes and alkynes are not.arrow_forward14-23 Arrange these compounds in order of increasing boiling point. Values in °C are 0, 35, and 97. CH3CH2CH2OH CH3CH2OCH2CH3 ch3ch2ch2ch3arrow_forward
- 14-66 1,4-Butanediol, hexane, and 1-pentanol have similar molecular weights. Their boiling points, arranged from lowest to highest, are 69°C, 138°C, and 230°C. Which compound has which boiling point?arrow_forward16-6 Answer true or false. te/7-Butylamine is a 3° amine. In an aromatic amine, one or more of the groups bonded to nitrogen is an aromatic ring. In a heterocyclic amine, the amine nitrogen is one of the atoms of a ring. The Lewis structures of both NH4~ and CH4show the same number (eight) of valence electrons, and the VSEPR model predicts tetrahedral geometry for each. There are four constitutional isomers with the molecular formula CgH^N.arrow_forward14-44 Answer true or false. (a) The functional group of a thiol is the —SH (sulfhydryl, group. (b, The parent name of a thiol is the name of the Ion gest carbon chain that contains the —SH group. (c) The S—H bond is nonpolar covalent. (d, The acidity of ethanethiol is comparable to that of phenol. Both phenols and thiols are classified as weak adds. The most common biological reaction of thiols is their oxidation to disulfides. The functional group of a disulfide is the —S—S— group. (h, Conversion of a thiol to a disulfide is a reduction reaction.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
