
Concept explainers
Each of the following compounds is characterized by a 1H NMR spectrum that consists of
only a single peak having the chemical shift indicated. Identify each compound.
Interpretation:
The compounds gives only single peak in 1H NMR spectrum as indicated by the chemical shift is to be identified.
Concept introduction:
The information related to the proton in the compound can be deduced by the number of signals in a spectrum.
The proton chemical shift in the compound is due to the environment of the proton.
The signal of a particular proton can be split by the presence of protons in vicinal position.
The NMR spectrum of a compound will consist of a single peak if all protons in the compound are structurally equivalent.
Index of hydrogen deficiency is calculated as
Here
Oxygen atoms do not affect the index of hydrogen deficiency.
Answer to Problem 31P
Solution:
Explanation of Solution
Chemical formula:
A single peak at
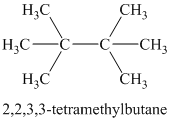
Therefore, the structure of the compound is:
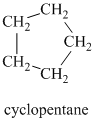
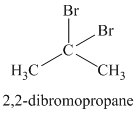
Chemical formula:
A single peak at
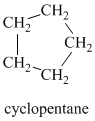
Therefore, it must be a symmetric cycloalkane:
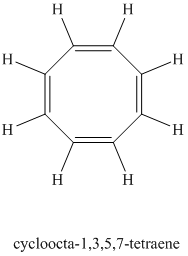
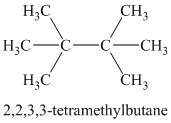
Chemical formula:
A single peak at
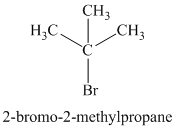
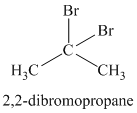
Chemical formula:
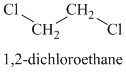
The chemical formula shows it is a saturated alkyl halide. A single peak means that all protons are equivalent. The presence of the bromine atoms accounts for the higher chemical shift of the protons. Therefore, the structure of the compound is:
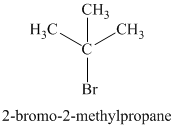
Chemical formula:
A single peak shows all protons to be equivalent. The formula is of a saturated alkyl halide. The presence of two chlorine atoms on the same carbon as the protons will increase the shift to
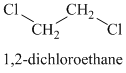
Chemical formula:
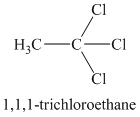
A single peak means all protons must be equivalent. The chemical formula shows it to be a saturated alkyl halide. The higher shift of
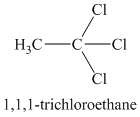
Chemical formula:
A single peak shows all protons to be equivalent. The chemical formula shows it to be a saturated alkyl halide. The high chemical shift of
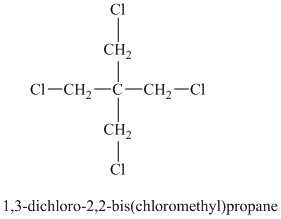
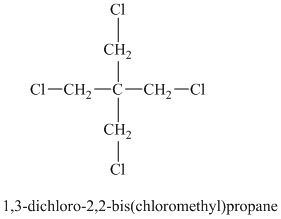
Chemical formula:
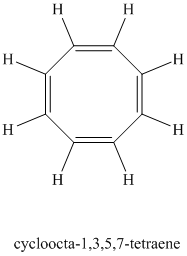
A single peak shows all protons are equivalent. The chemical formula shows an index of hydrogen deficiency of four. This, taken together with a chemical shift of
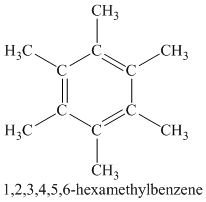
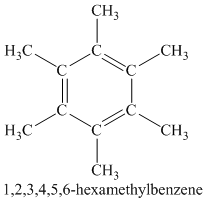
Chemical formula:
A single peak shows all protons are equivalent. The higher chemical shift of
Therefore, the structure of the compound is:
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- The 1H NMR spectrum of CH3OH recorded on a 500 MHz NMR spectrometer consists of two signals, one due to the CH3 protons at 1715 Hz and one due to the OH proton at 1830 Hz, both measured downeld from TMS. (a) Calculate the chemical shift of each absorption. (b) Do the CH3 protons absorb upeld or downeld from the OH proton?arrow_forwardIdentify A and B, isomers of molecular formula C3H4Cl2, from the given 1H NMR data: Compound AJ = 6.9 Hz) and 5.89 (quartet, 1 H, J = 6.9 Hz) ppm. Compound B exhibits signals at 4.16 (singlet, 2 H), 5.42 (doublet, 1 H, J = 1.9 Hz), and 5.59 (doublet, 1 H, J = 1.9 Hz) ppm.arrow_forwardCompound A exhibits two signals in its 1H NMR spectrum at 2.64 and 3.69 ppm, and the ratio of the absorbing signals is 2:3. Compound B exhibits two signals in its 1H NMR spectrum at 2.09 and 4.27 ppm, and the ratio of the absorbing signals is 3:2. Which compound corresponds to dimethyl succinate, and which compound corresponds to ethylene diacetate?arrow_forward
- Identify A and B, isomers of molecular formula C3H4Cl2, from the given 1H NMR data: Compound A exhibits peaks at 1.75 (doublet, 3 H, J = 6.9 Hz) and 5.89 (quartet, 1 H, J = 6.9 Hz) ppm. Compound B exhibits peaks at 4.16 (singlet, 2 H), 5.42 (doublet, 1 H, J = 1.9 Hz), and 5.59 (doublet, 1 H, J = 1.9 Hz) ppm.arrow_forwardCompound B has molecular formula C9H12. It shows five signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.22 ppm, a septet of integral 1 at 2.86 ppm, a singlet of integral 1 at 5.34 ppm, a doublet of integral 2 at 6.70 ppm, and a doublet of integral 2 at 7.03 ppm. The 13C-NMR spectrum of B shows six unique signals (23.9, 34.0, 115.7, 128.7, 148.9, and 157.4). Identify B and explain your reasoning.arrow_forwardIdentify each of the following compounds from the 1H NMR data and molecular formula:a. C4H8Br2: a 6H singlet at 1.97 ppma 2H singlet at 3.89 ppm b. C8H9Br: a 3H doublet at 2.01 ppma 1H quartet at 5.14 ppma 5H broad singlet at 7.35 ppm c. C5H10O2: a 3H triplet at 1.15 ppma 3H triplet at 1.25 ppma 2H quartet at 2.33 ppma 2H quartet at 4.13 ppmarrow_forward
- Which compounds give a 1H NMR spectrum with two signals in a ratio of 2:3?arrow_forwardThe 1H-NMR spectrum of the compound below shows signals at 1.33, 2.70, 3.84, and 7.27 ppm. Assign the correct chemical shift to each proton environment.arrow_forwardThe 1H-NMR spectrum of Compound C shows five signals – δ 2.38 (1H, dt), 2.72 (1H, dt), 5.34 (1H, t), 5.49 (2H, ddd), 6.27 (2H, dd) ppm. Its 13C-NMR spectrum has four signals – δ 26, 58, 127, 129 ppm. In the compound’s mass spectrum, the M+1 peak appears at m/z = 115. An M+2 peak, whose intensity is roughly one-third that of the M+1 peak, also appears. Suggest a structure for this compound.arrow_forward
- Which of the following structures would have fewer 13C signals than 1H signals in its NMR spectra? a) 1 b) 2 c) 3 d) 4 e) 5arrow_forwardWhich one of the following isomeric C8H18 compounds has five peaks in its 13C NMR spectrum?arrow_forwardThe compound has a molecular formula of C5H10O and the following 13C spectrum. A proton NMR was measured, but the data was lost except for the fact that it showed three distinct signals: a singlet, a doublet, and a septet, all of which appear between 0 and 3 ppm. Identify the compound, and draw its 1H NMR.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





