
Concept explainers
a) 1 mol Br2 in CH2Cl2
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are to be given.
Concept introduction:
Conjugated dienes are treated with bromine in CH2Cl2 undergo both 1,2 and 1,4 addition of bromine to yield different products.
To give:
The product expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2.
Answer to Problem 27AP
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are 3,6-dibromocyclohexene and 3,4-dibromocyclohexene.

Explanation of Solution
1,3-cyclohexadiene is a conjugated diene. When treated with one mole of bromine in CH2Cl2 it yields 3,6-dibromocyclohexene by 1,4 addition and 3,4-dibromocyclohexene by 1,2 addition reaction.
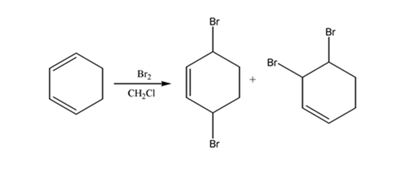
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are 3,6-dibromocyclohexene and 3,4-dibromocyclohexene.

b) O3 followed by Zn
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc is to be given.
Concept introduction:
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc is to be given.
Answer to Problem 27AP
The products expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc are glyoxal and succindialdehyde.
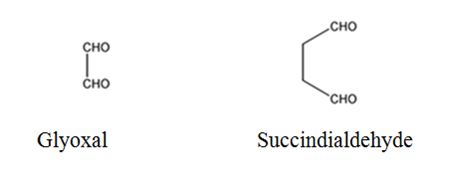
Explanation of Solution
Ozone adds to both the double bonds in 1,3-cyclohexadiene to form an ozonide. When treated with Zn and acetic acid the ozonide breaks to yield carbonyl compounds. The carbons that have oxygen in the products are joined through double bonds in the reactant.
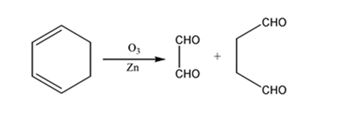
The products expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc are glyoxal and succindialdehyde.

c) 1 mol HCl in ether
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl in ether is to be given.
Concept introduction:
Conjugated dienes when treated with one mole of HCl in ether undergo both 1,2 and 1,4 addition of HCl to yield different products. With unsubstituted cyclic conjugated 1,3-dienes a single product is produced in both types of addition.
To give:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is to be given.
Answer to Problem 27AP
The product expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is 3-chlorocyclohexene.
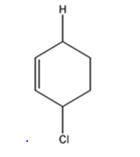
Explanation of Solution
Both 1,2 and 1,4 addition of HCl to 1,3-cyclohexadiene leads to the formation of the same product, 3-chlorocyclohexene, as it is an unsubstituted conjugated 1,3-diene.
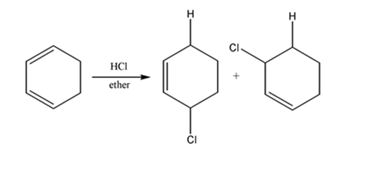
The product expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is 3-chlorocyclohexene.
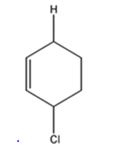
d) 1 mol DCl in ether
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are to be given.
Concept introduction:
Conjugated dienes when treated with one mole of DCl in ether undergo both 1,2 and 1,4 addition of DCl to yield different products.
To give:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl.
Answer to Problem 27AP
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are 3-chloro-4-deuteratedcyclohexene and 3-chloro-6-deuteratedcyclohexene.
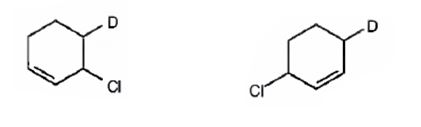
Explanation of Solution
1,3-cyclohexadiene is a conjugated diene. When treated with one mole of DCl it yields 3-chloro-4-deuteratedcyclohexen by 1,2 addition 3-chloro-6-deuteratedcyclohexene by 1,4 addition reaction.
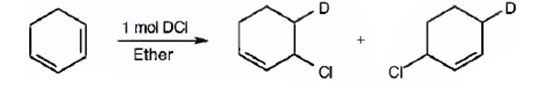
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are 3-chloro-4-deuteratedcyclohexene and 3-chloro-6-deuteratedcyclohexene.

e) 3-Buten-2-one (H2C=CHCOCH3)
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is to be given.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. During the reaction the diene and dienophile orient themselves on top of one another and so an endo product results.
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one.
Answer to Problem 27AP
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is
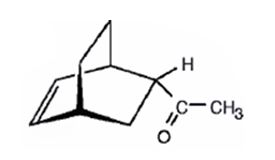
Explanation of Solution
The diene, 1,3-cyclohexadiene and the dienophile 3-buten-2-one arrange themselves one on the top of the other and react through the formation of a cyclic six member transition state to yield the endo product.
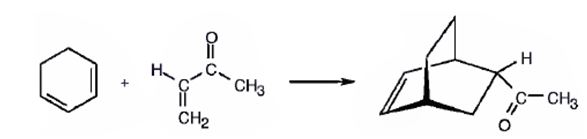
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is
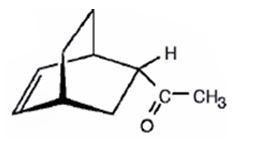
f) Excess OsO4, followed by NaHSO3
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is to be given.
Concept introduction:
The reaction given involves hydroxylation of the double bonds in the diene. When a diene is treated with OsO4, a single step addition of OsO4 to each of the double bonds takes place to give a cyclic osmate. The cyclic osmate gets cleaved when treated with NaHSO3 yields a tetraol.
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3.
Answer to Problem 27AP
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is cyclohexane-1,2,3,4-tetraol.
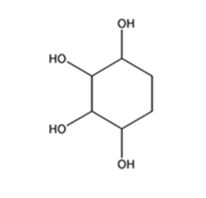
Explanation of Solution
When 1,3-cyclohexadiene is treated with excess OsO4 the addition of OsO4 takes place to both the double bonds in it. The addition occurs with syn stereochemistry to yield a cyclic osmate. The cyclic osmate then gets cleaved when treated with NaHSO3 to yield the tetraol.
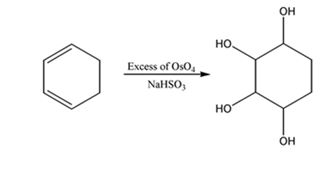
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is cyclohexane-1,2,3,4-tetraol.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- Following are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives which alkene? Account for the stereoselectivity of each -elimination.arrow_forwardCompound X, C7H15Cl, is a chiral product of the radical chlorination of 2-methylhexane.Base-promoted E2 elimination of X gives a single product.What is the structure of X?arrow_forwardAs an example of a ver rare occurence, the following hydrocarbon reacts with two equivalents of butylithium to form a dianion of the formula [C8H6]2- . Propose a structure for this dianion and explain why this dianion forms so readily.arrow_forward
- 5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardCompound A has molecular formula C4H10, and gives two monochlorides, B and C, on photochemical chlorination. Treatment of either of these monochlorides with potassium tert-butoxide gives the same alkene (C4H8) as the product, but B leads to just one isomer of the alkene, D, where C gives D and another isomer of the alkene, E. Treatment of monochlorides B and C with aqueous ethanol gives products F and G, respectively, both of which are of molecular formula C4H10O. What are the names of compounds A-G?arrow_forwardAddition of HBr to 3,3-dimethyl-1-butene gives a mixture of two isomeric alkyl bromide products. Draw structures for the two products, and give a mechanistic explanation for their formation.arrow_forward
- Compound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr), which reacts violently with D2O togive 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (CH3COCH3)followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2SO4 gives E (C10H16), which decolorizestwo equivalents of Br2 to give F (C10H16Br4). E undergoes hydrogenation with excess H2 and a Pt catalyst to giveisobutylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughoutarrow_forwardOzonolysis (O3 in CH2Cl2) of compound A under reducing conditions (Zn /acetic acid) gives formaldehyde, 2-butanone, and compound B. Catalytic hydrogenation (H2/Pd) of A gives 2,7-dimethylnonane. What is a possible structure for compound A? a. 2,7-Dimethyl-2,8-nonadieneb. 2,7-Dimethyl-1,8-nonadienec. 2,7-Dimethyl-1,6-nonadiened. 2,7-Dimethyl-1,7-nonadienearrow_forwardDeduce the structure of each compound from the information given. All unknowns in this problem have molecularformula C8H12.(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC¬(CH2)6¬COOH. Draw the structure of Warrow_forward
- When 5-bromo-1-pentanol is treated with sodium hydride in diethyl ether, the product is analyzed to be C5H10O. Propose a likely structure for this product, suggesting a reasonable mechanistic pathway for its formationarrow_forwardalkylation of benzene with 1-chlorobutane in the presence of AlCl3 gives both butylbenzene and (1-methylpropyl)benzene as products. Propose a route to butylbenzene from benzene that does not also give the (1-methylpropyl)benzene side-product.arrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr), which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (CH3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2 SO4 gives E (C10 H16), which decolorizes two equivalents of Br2 to give F (C10H16 Br4). E undergoes hydrogenation with excess H2 and a Pt catalyst to give isobutylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
