
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 14PS
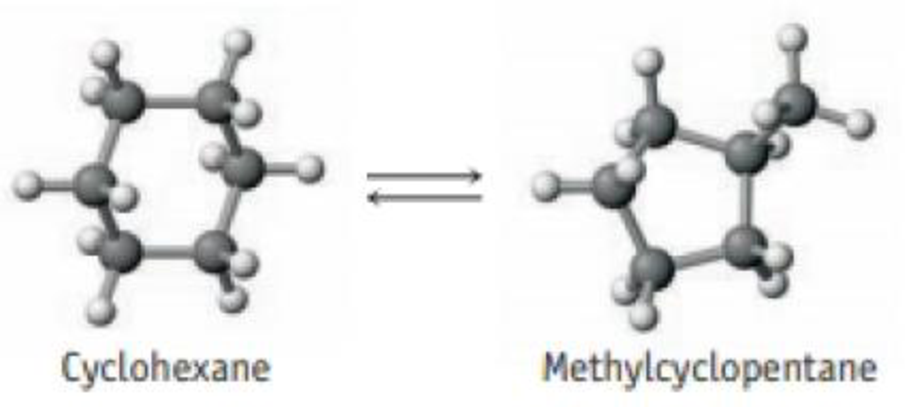
Cyclohexane, C6H12, a hydrocarbon, can isomerize or change into methylcyclopentane, a compound of the same formula (C5H9CH3) but with a different molecular structure.
 sssss
sssss
The equilibrium constant has been estimated to be 0.12 at 25 °C. If you had originally placed 0.045 mol of cyclohexane in a 2.8-L flask, what would be the concentrations of cyclohexane and methylcyclopentane when equilibrium is established?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 15 Solutions
Chemistry & Chemical Reactivity
Ch. 15.2 - Write the equilibrium constant expression for each...Ch. 15.2 - Answer the following questions regarding the...Ch. 15.3 - A solution is prepared by dissolving 0.050 mol of...Ch. 15.4 - At some temperature. Kc = 33 for the reaction...Ch. 15.4 - The decomposition of PCl5(g) to form PCl3(g) and...Ch. 15.5 - The conversion of oxygen to ozone has a very small...Ch. 15.6 - Equilibrium exists between butane and isobutane...Ch. 15.6 - Anhydrous ammonia is used directly as a...Ch. 15.6 - Prob. 1.2ACPCh. 15.6 - Freezing point depression is one means of...
Ch. 15.6 - Prob. 2.2ACPCh. 15.6 - A 0.64 g sample of the white crystalline dimer (4)...Ch. 15.6 - Predict whether the dissociation of the dimer to...Ch. 15.6 - Prob. 2.5ACPCh. 15 - Write equilibrium constant expressions for the...Ch. 15 - Write equilibrium constant expressions for the...Ch. 15 - Kc = 5.6 1012 at 500 K for the dissociation of...Ch. 15 - The reaction 2 NO2(g) N2O4(g) has an equilibrium...Ch. 15 - A mixture of SO2, O2, and SO3 at 1000 K contains...Ch. 15 - The equilibrium constant Kc, for the reaction 2...Ch. 15 - The reaction PCl5(g) PCl3(g) + Cl2(g) was...Ch. 15 - An equilibrium mixture of SO2, O2, and SO3 at a...Ch. 15 - The reaction C(s) + CO2(g) 2 CO(g) occurs at high...Ch. 15 - Hydrogen and carbon dioxide react at a high...Ch. 15 - A mixture of CO and Cl2 is placed in a reaction...Ch. 15 - You place 0.0300 mol of pure SO3 in an 8.00-L...Ch. 15 - The value of Kc for the interconversion of butane...Ch. 15 - Cyclohexane, C6H12, a hydrocarbon, can isomerize...Ch. 15 - The equilibrium constant for the dissociation of...Ch. 15 - The equilibrium constant, Kc, for the reaction...Ch. 15 - Carbonyl bromide decomposes to carbon monoxide and...Ch. 15 - Iodine dissolves in water, but its solubility in a...Ch. 15 - Which of the following correctly relates the...Ch. 15 - Which of the following correctly relates the...Ch. 15 - Consider the following equilibria involving SO2(g)...Ch. 15 - The equilibrium constant K for the reaction CO2(g)...Ch. 15 - Calculate K for the reaction SnO2(s) + 2 CO(g) ...Ch. 15 - Calculate K for the reaction Fe(s) + H2O(g) ...Ch. 15 - Relationship of Kc and Kp: (a) Kp for the...Ch. 15 - Relationship of Kc and Kp: (a) The equilibrium...Ch. 15 - Dinitrogen trioxide decomposes to NO and NO2, in...Ch. 15 - Kp for the following reaction is 0.16 at 25 C: 2...Ch. 15 - Consider the isomerization of butane with an...Ch. 15 - The decomposition of NH4HS NH4HS(s) NH3(g) +...Ch. 15 - Suppose 0.086 mol of Br2 is placed in a 1.26-L...Ch. 15 - The equilibrium constant for the reaction N2(g) +...Ch. 15 - Kp for the formation of phosgene, COCl2, is 6.5 ...Ch. 15 - The equilibrium constant, Kc, for the following...Ch. 15 - Carbon tetrachloride can be produced by the...Ch. 15 - Equal numbers of moles of H2 gas and I2 vapor are...Ch. 15 - The equilibrium constant for the butane isobutane...Ch. 15 - At 2300 K the equilibrium constant for the...Ch. 15 - Which of the following correctly relates the two...Ch. 15 - Consider the following equilibrium: COBr2(g) ...Ch. 15 - Heating a metal carbonate leads to decomposition....Ch. 15 - Phosphorus pentachloride decomposes at elevated...Ch. 15 - Ammonium hydrogen sulfide decomposes on heating....Ch. 15 - Ammonium iodide dissociates reversibly to ammonia...Ch. 15 - When solid ammonium carbamate sublimes, it...Ch. 15 - In the gas phase, acetic acid exists as an...Ch. 15 - Assume 3.60 mol of ammonia is placed in a 2.00-L...Ch. 15 - The total pressure for a mixture of N2O4 and NO2...Ch. 15 - Kc for the decomposition of ammonium hydrogen...Ch. 15 - Prob. 52GQCh. 15 - A 15-L flask at 300 K contains 6.44 g of a mixture...Ch. 15 - Lanthanum oxalate decomposes when heated to...Ch. 15 - The reaction of hydrogen and iodine to give...Ch. 15 - Sulfuryl chloride, SO2Cl2 is used as a reagent in...Ch. 15 - Hemoglobin (Hb) can form a complex with both O2...Ch. 15 - Limestone decomposes at high temperatures....Ch. 15 - At 1800 K, oxygen dissociates very slightly into...Ch. 15 - Nitrosyl bromide, NOBr, dissociates readily at...Ch. 15 - A Boric acid and glycerin form a complex...Ch. 15 - The dissociation of calcium carbonate has an...Ch. 15 - A sample of N2O4 gas with a pressure of 1.00 atm...Ch. 15 - Prob. 64GQCh. 15 - The photograph below shows what occurs when a...Ch. 15 - The photographs below (a) show what occurs when a...Ch. 15 - Decide whether each of the following statements is...Ch. 15 - Neither PbCl2 nor PbF2 is appreciably soluble in...Ch. 15 - Characterize each of the following as product- or...Ch. 15 - The size of a flask containing colorless N2O4(g)...Ch. 15 - Describe an experiment that would allow you to...Ch. 15 - The chapter opening photograph (page 670) showed...Ch. 15 - Suppose a tank initially contains H2S at a...Ch. 15 - Pure PCl5 gas is placed in a 2.00-L flask. After...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nitrosyl chloride, NOC1, decomposes to NO and Cl2 at high temperatures. 2 NOCl(g) ⇌ 2 NO(g) + Cl2(g) Suppose you place 2.00 mol NOC1 in a 1.00–L flask, seal it, and raise the temperature to 462 °C. When equilibrium has been established, 0.66 mol NO is present. Calculate the equilibrium constant Kc for the decomposition reaction from these data.arrow_forwardA mixture of 0.0565 mol phosphorus pentachloride, PCl5, and 0.0800 mol helium gas, He, was placed in a 1.000-L flask and heated to 250.0C. The phosphorus pentachloride decomposes at this temperature to give phosphorus trichloride, PCl3, and chlorine gas, Cl2. The helium gas is inert. PCl5(g)PCl3(g)+Cl2(g) What is the partial pressure of helium in this equilibrium mixture at 250.0C? At equilibrium, the total pressure is found to be 6.505 atm. What is Kc for the dissociation of PCl5?arrow_forwardTwo molecules of A react to form one molecule of B, as in the reaction 2 A(g) B(g) Three experiments are done at different temperatures and equilibrium concentrations are measured. For each experiment, calculate the equilibrium constant, Kc. (a) [A] = 0.74 mol/L, [B] = 0.74 mol/L (b) [A] = 2.0 mol/L, [B] = 2.0 mol/L (c) [A] = 0.01 mol/L, [B] = 0.01 mol/L What can you conclude about this statement: If the concentrations of reactants and products are equal, then the equilibrium constant is always 1.0.arrow_forward
- Gaseous acetic acid molecules have a certain tendency to form dimers. (A dimer is a molecules formed by the association of two identical, simpler molecules.) The equilibrium constant Kp at 25C for this reaction is 1.3 103. a If the initial pressure of CH3COOH monomer (the simpler molecule) is 7.5 103 atm, what are the pressures of monomer and dimer when the system comes to equilibrium? (The simpler quadratic equation is obtained by assuming that all of the acid molecules have dimerized and then some of it dissociates to monomer.) b Why do acetic acid molecules dimerize? What type of structure would you draw for the dimer? c As the temperature decreases, would you expect the percentage of dimer to increase or decrease? Why?arrow_forwardMethanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Once in the body, the substance is oxidized to produce formaldehyde (embalming fluid) and eventually formic acid. Both of these substances are also toxic in varying levels. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq) Assuming the value of K for this reaction is 3.7 1010, what are the equilibrium concentrations of each species if you start with a 1.24 M solution of methanol? What will happen to the concentration of methanol as the formaldehyde is further converted to formic acid?arrow_forwardCarbon dioxide reacts with carbon to give carbon monoxide according to the equation C(s)+CO2(g)2CO(g) At 700. C, a 2.0-L sealed flask at equilibrium contains 0.10 mol CO, 0.20 mol CO2, and 0.40 mol C. Calculate the equilibrium constant KP for this reaction at the specified temperature.arrow_forward
- Dinitrogen tetroxide, N2O4, is a colorless gas (boiling point, 21C), which dissociates to give nitrogen dioxide, NO2 a reddish brown gas. N2O4(g)2NO2(g) The equilibrium constant Kc at 25C is 0.125. What percentage of dinitrogen tetroxidc is dissociated when 0.0400 mol N2O4 is placed in a 1.00-L flask at 25C?arrow_forwardAt room temperature, the equilibrium constant Kc for the reaction 2 NO(g) ⇌ N2(g) + O2(g) is 1.4 × 1030. Is this reaction product-favored or reactant-favored? Explain your answer. In the atmosphere at room temperature the concentration of N2 is 0.33 mol/L, and the concentration of O2 is about 25% of that value. Calculate the equilibrium concentration of NO in the atmosphere produced by the reaction of N2 and O2. How does this affect your answer to Question 11?arrow_forwardGaseous acetic acid molecules have a certain tendency to form dimers. (A dimer is a molecule formed by the association of two identical, simpler molecules.) The equilibrium constant Kc at 25C for this reaction is 3.2 104. a If the initial concentration of CH3COOH monomer (the simpler molecule) is 4.0 104 M, what are the concentrations of monomer and dimer when the system comes to equilibrium? (The simpler quadratic equation is obtained by assuming that all of the acid molecules have dimerized and then some of it dissociates to monomer.) b Why do acetic acid molecules dimerize? What type of structure would you draw for the dimer? c As the temperature increases would you expect the percentage of dimer to increase or decrease? Why?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY