
Concept explainers
Draw all constitutional isomers formed when each
a.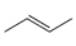 b.
b.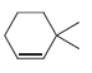 c.
c. 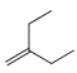
(a)
Interpretation: The constitutional isomer formed by the reaction of given alkene with
Concept introduction: The reaction of alkene with
Answer to Problem 15.18P
The constitutional isomers formed by the reaction of given alkene with
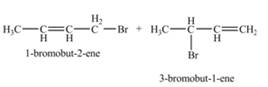
Explanation of Solution
The reaction of alkene with
The given alkene is shown below.
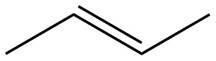
Figure 1
The general steps involved in the reaction of allylic bromination with
• The first step is the initiation of bromine radical.
• The next step is the propagation in which generation of allylic radical take place.
• In third step, allylic radical reacts with
The constitutional isomer formed by the reaction of given alkene with
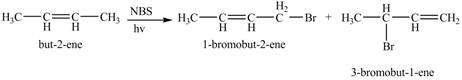
Figure 2
Therefore, the constitutional isomers formed for the given reaction are
The constitutional isomers formed by the reaction of given alkene with
(b)
Interpretation: The constitutional isomer formed by the reaction of given alkene with
Concept introduction: The reaction of alkene with
Answer to Problem 15.18P
The constitutional isomers formed by the reaction of given alkene with
Explanation of Solution
The reaction of alkene with
The given alkene is shown below.
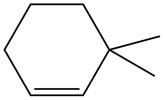
Figure 3
The general steps involved in the reaction of allylic bromination with
• The first step is the initiation of bromine radical.
• The next step is the propagation in which generation of allylic radical take place.
• In third step, allylic radical reacts with
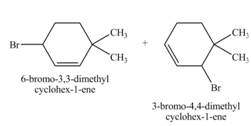
The constitutional isomer formed by the reaction of given alkene with
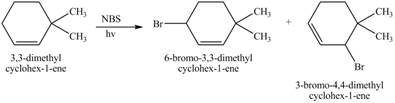
Figure 4
Therefore, the constitutional isomers formed for the given reaction are
The constitutional isomers formed by the reaction of given alkene with
(c)
Interpretation: The constitutional isomer formed by the reaction of given alkene with
Concept introduction: The reaction of alkene with
Answer to Problem 15.18P
The constitutional isomers formed by the reaction of given alkene with
Explanation of Solution
The reaction of alkene with
The given alkene is shown below.
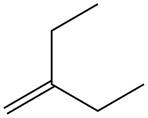
Figure 5
The general steps involved in the reaction of allylic bromination with
• The first step is the initiation of bromine radical.
• The next step is the propagation in which generation of allylic radical take place.
• In third step, allylic radical reacts with
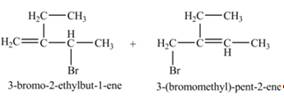
The constitutional isomer formed by the reaction of given alkene with
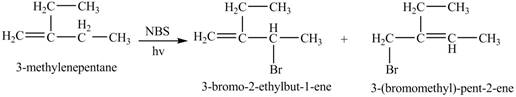
Figure 6
Therefore, the constitutional isomers formed for the given reaction are
The constitutional isomers formed by the reaction of given alkene with
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- Draw a stepwise mechanism for the following isomerization.arrow_forwardA is a toxin produced by the poisonous seaweed Chlorodesmis fastigiata. (a) Label each alkene that exhibits stereoisomerism as E or Z.arrow_forwardHow is each compound related to A? Choose from tautomers, constitutional isomers but not tautomers, or neither.arrow_forward
- Draw the structure of A,B,and Carrow_forward(a) What is the major alkene formed when A is dehydrated with H2SO4? (b) What is the major alkene formed when A is treated with POCl3 and pyridine? Explain why the major product is different in these reactions.arrow_forwardDraw all constitutional isomers formed when X is treated with NBS + hν.arrow_forward
- Draw the products (including stereoisomers) formed when benzaldehyde (C6H5CHO) is treated with each Wittig reagent.arrow_forwardβ-D-Glucose, a hemiacetal, can be converted to a mixture of acetals on treatment with CH3OH in the presence of acid. Draw a stepwise mechanism for this reaction. Explain why two acetals are formed from a single starting material.arrow_forwardDraw a stepwise mechanism for the following reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single step.arrow_forward
- Draw the products formed when A is treated with each reagent: (a) H2 + Pd-C; (b) mCPBA; (c) PCC; (d) CrO3, H3SO4, H2O; (e) Sharpless reagent with (+)-DET.arrow_forwardDraw a stepwise mechanism for the attached reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single steparrow_forwardDraw a stepwise mechanism for the following reaction that forms ether D. D can be converted to the antidepressant uoxetine (trade name Prozac) in a single step.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





