
Concept explainers
Interpretation:
The structural features of the
Concept introduction:
A carbonyl group has a carbon atom that is connected to an oxygen atom through a double bond and single bonded to two other carbon atoms respectively.
•
• The alcohols contain hydroxyl
• The carboxylic acids contain carboxylic
Answer to Problem 15.1E
The structure of carboxylic acid functional group is shown below.
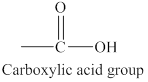
The structure of carboxylic acid functional group is different from alcohols, aldehydes or ketones because it possesses both carbonyl group and hydroxyl group. On the other hand, alcohols has only hydroxyl group and aldehydes or ketones contain only carbonyl group in their structures.
Explanation of Solution
The carboxylic acid functional group has a carbon atom bonded to an oxygen atom by a double bond and bonded to a hydroxyl group
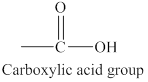
Figure 1
Aldehydes and ketones contain carbonyl functional group in their parent chain. The structural formula of an aldehyde is
The structure of carboxylic acid functional group is different from both aldehydes and ketones because it has hydroxyl group in addition to the carbonyl group.
The alcohols contain only hydroxyl
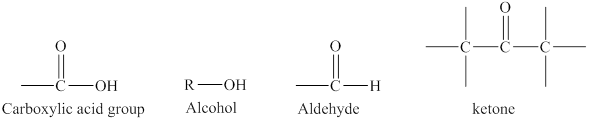 Figure 2
Figure 2
The carboxylic acid functional group structure is shown in Figure 1.
The difference in the structure of carboxylic acid from the structures of an alcohol, an aldehyde or a ketone is rightfully stated.
Want to see more full solutions like this?
Chapter 15 Solutions
Chemistry for Today: General, Organic, and Biochemistry
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





