
Concept explainers
(a)
Interpretation: The given radical is to be classified as
Concept introduction: A free radical is an atom or ion with unpaired electron. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.1P
The given free radical is classified as
Explanation of Solution
The given species is,
![]()
Figure 1
The given free radical is attached to two carbon atoms. Thus, it is classified as
The given free radical is classified as
(b)
Interpretation: The given radical is to be classified as
Concept introduction: A free radical is an atom or ion with unpaired electron. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.1P
The given free radical is classified as
Explanation of Solution
The given species is,
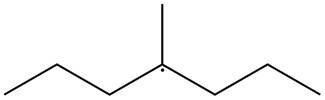
Figure 2
The given free radical is attached to three carbon atoms. Thus, it is classified as
The given free radical is classified as
(c)
Interpretation: The given radical is to be classified as
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.1P
The given free radical is classified as
Explanation of Solution
The given species is,
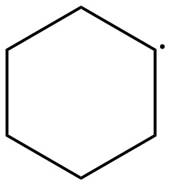
Figure 3
The given free radical is attached to two carbon atoms. Thus, it is classified as
The given free radical is classified as
(d)
Interpretation: The given radical is to be classified as
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.1P
The given free radical is classified as
Explanation of Solution
The given species is,
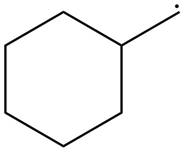
Figure 4
The given free radical is attached to one carbon atom. Thus, it is classified as
The given free radical is classified as
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEM W/CONNECT & MODEL KIT >CI
- will vote thumb up for the correct answer! plIdentify the best reagents to complete the following reaction.arrow_forwardDraw the products of each reaction. (a) and (b)arrow_forwardResveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.arrow_forward
- Rank the attached carbocations in order of increasing stability?arrow_forwardWhat is the correct witig product?arrow_forwardDraw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.arrow_forward
- Which group in following pair is assigned the higher priority? −H, −Darrow_forwardA. Write appropriate reagents over the reaction arrow B. As a result of this reaction, is the N made more nucleophilic or less nucleophilic?arrow_forwardpls draw a stepwise mechanism for the reaction thanks!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
