
Concept explainers
(a)
Interpretation:
The target molecule transformation should be draw and identified given the starting molecule of
Concept Introduction:
Oxidation Reaction: Generally alcohol can be oxidizing to aldehyde or
Reduction Reaction: This process in which any substance atoms, ion or molecule gains one or more electrons it is called reduction.
(a)
Answer to Problem 15.20UKC
The hydride added to the carbonyl carbon, the polar (C=O) carbon has a partial positive charge, then the reduction process has
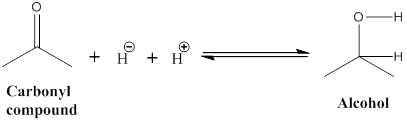
Explanation of Solution
Reduction reaction: The given carbonyl molecule is converted into alcohol (b) in this undergoes for reduction process was occurred. The hydride ion added to the carbonyl carbon, because carbonyl carbon is polar it as partial positive charges in carbon atom.
(b)
To determine give the reactions indicate which direction represents reduction and which represents oxidation.
Concept Introduction:
Carbonyl group: This group presence of a
Oxidation Reaction: Generally alcohol can be oxidizing to aldehyde or ketone and aldehyde can be further oxidized to carboxylic acids.
Reduction Reaction: This process in which any substance atoms, ion or molecule gains one or more electrons it is called reduction.
(b)
Answer to Problem 15.20UKC
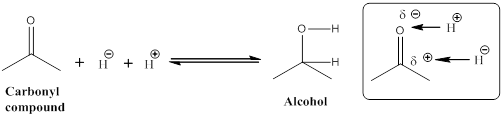
Given the arrow to the right represents reduction and the arrow to the left represents oxidation.
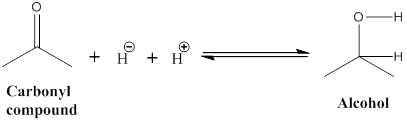
Explanation of Solution
Let us consider given reaction,

Given the arrow to the right represents reduction and the arrow to the left represents oxidation.
Want to see more full solutions like this?
Chapter 15 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText -- ValuePack ... and Biological Chemistry (4th Edition)
- In the preparation of aspirin, You can do the functional group test to ensure the completion of the reaction. (a) What is the name of reagent used? (b) What is your observation if any unreacted starting material is present? (c) What is the name of the functional group responsible for this reaction?arrow_forwardwhich statement is true for the following reaction?arrow_forwardWhich concept is illustrated by the reaction shown in the diagram?arrow_forward
- discuss the following statement: “Whether the ΔG for a reaction is larger, smaller, or the same as ΔG° depends on the concentration of the compounds that participate in the reaction.”arrow_forwardIn the Equation: HCO3- + HCl --------> Conjugate Acid + Conjugate Base a ) Which reactant is the Acid? b) Which reactant is the Base? c) What Conjugate Acid will form in this reaction? d) What Conjugate Base will form in this reaction?arrow_forwardThe reaction CO (g) + H2O(g) CO2(g) 2 + H2(g) has ΔH = -41 kJ/mol. Does the amount of H2 in an equilibrium mixture increase or decrease when the temperature is decreased?arrow_forward
- Why is it unlikely that nonenzymatic catalysts operate by preferentially binding the transition state?arrow_forwardIdentify the oxidized reactant, the reduced reactant, the oxidizing agent, and the reducing agent in the following reactions:arrow_forwardWhat three important properties do catalyst have?arrow_forward
- if the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established A + B ⇌ C a. the concentration of C would increase for a time, then remain constant b. the concentration of A would increase for a time, then decrease c. the concentration of B would increase for a time, then remain constantarrow_forwardWhat structural feature is necessary for an alcohol to undergo oxidation reactions?arrow_forwardDecarboxylases can catalyze which type of reaction?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





