
(a)
Interpretation:
Molecular formula for 2-butanol has to be given.
Concept Introduction:
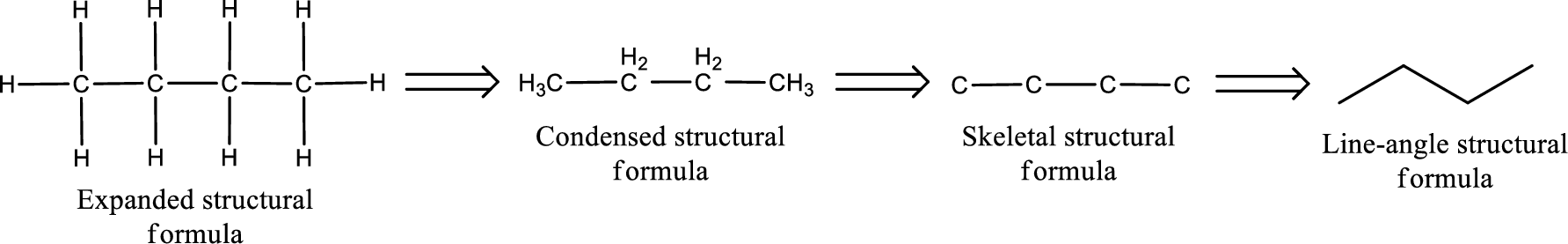
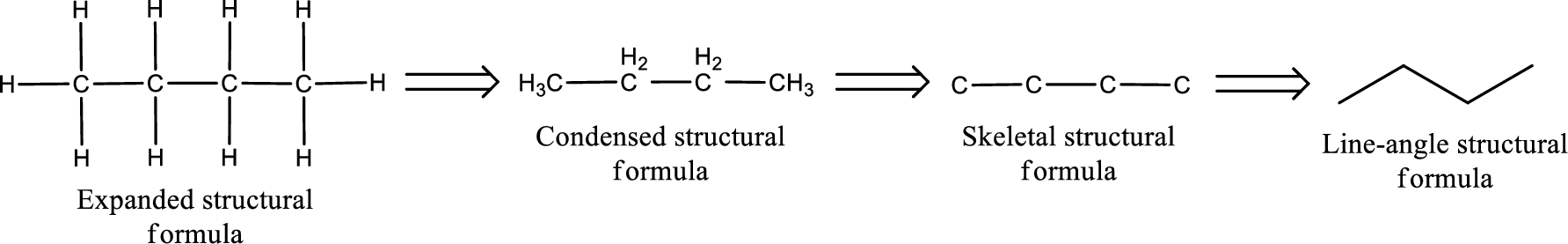
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
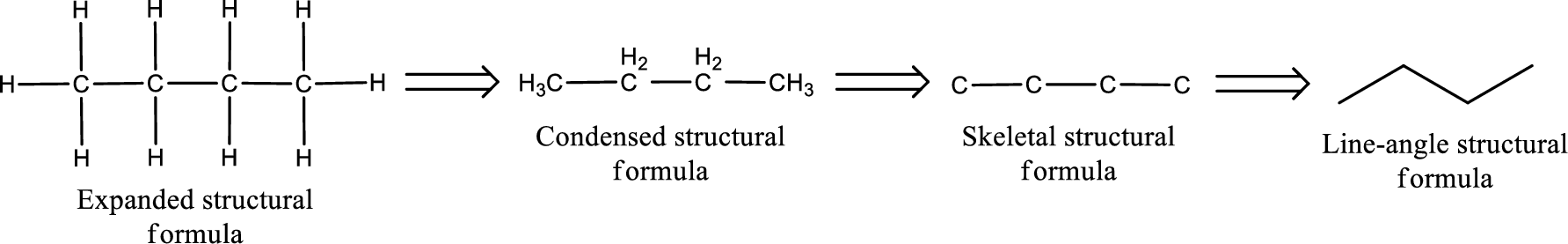
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(a)
Answer to Problem 15.47EP
Molecular formula of 2-butanol is
Explanation of Solution
The given name of the compound is 2-butanol. From the name it is understood that the parent carbon chain is butane and it contains four carbon atoms. The parent chain can be drawn as shown below,
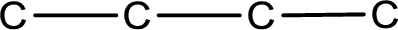
From the name of the given compound, it is identified to be an alcohol with the hydroxyl group present on the second carbon atom. The carbonyl carbon atom is the second carbon atom.
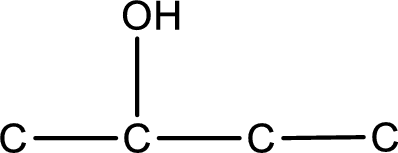
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
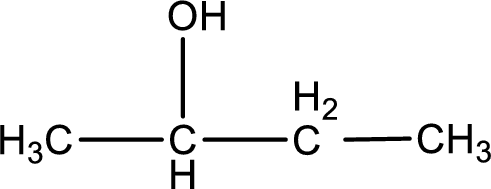
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of 2-butanol is given.
(b)
Interpretation:
Molecular formula for butanal has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(b)
Answer to Problem 15.47EP
Molecular formula of butanal is
Explanation of Solution
The given name of the compound is butanal. From the name it is understood that the parent carbon chain is butane and it contains four carbon atoms. The parent chain can be drawn as shown below,
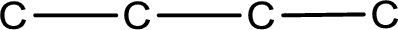
From the name of the given compound, it is identified to be an
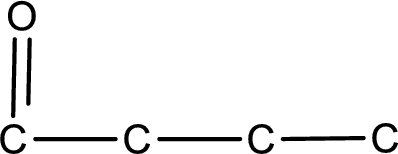
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
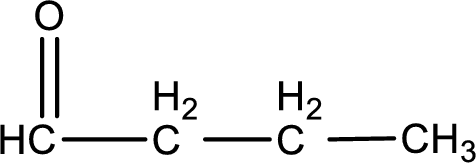
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of butanal is given.
(c)
Interpretation:
Molecular formula for butanone has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
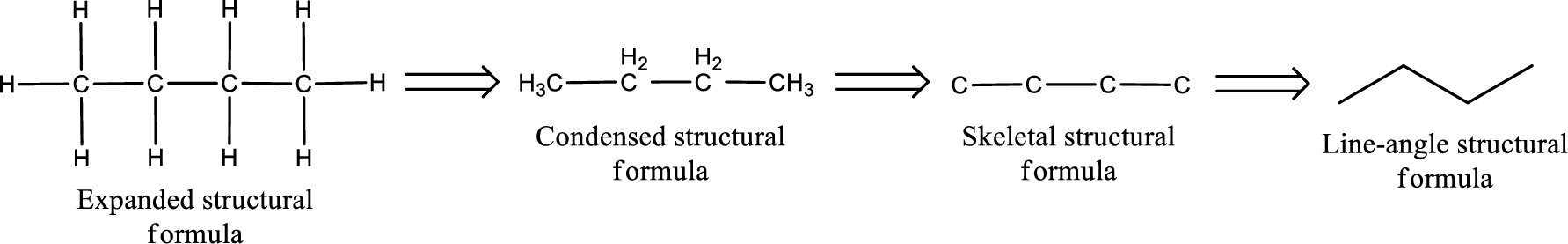
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(c)
Answer to Problem 15.47EP
Molecular formula of butanone is
Explanation of Solution
The given name of the compound is butanone. From the name it is understood that the parent carbon chain is butane and it contains four carbon atoms. The parent chain can be drawn as shown below,
From the name of the given compound, it is identified to be a
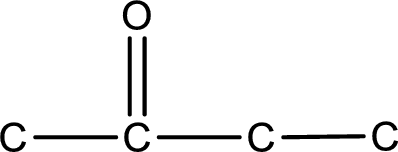
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
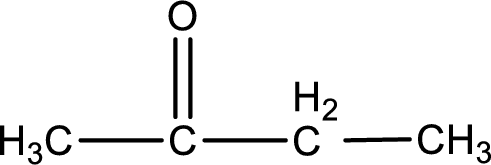
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of butanone is given.
(d)
Interpretation:
Molecular formula for butanedial has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(d)
Answer to Problem 15.47EP
Molecular formula of butanedial is
Explanation of Solution
The given name of the compound is butanedial. From the name it is understood that the parent carbon chain is butane and it contains four carbon atoms. The parent chain can be drawn as shown below,
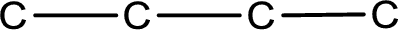
From the name of the given compound, it is identified to be an aldehyde. The carbonyl carbon atom is the first carbon atom and fourth carbon atom.
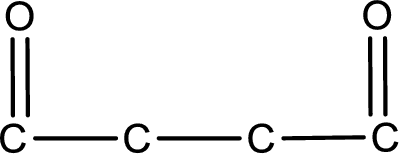
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
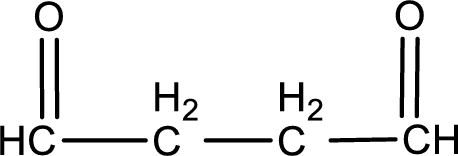
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of butanedial is given.
Want to see more full solutions like this?
Chapter 15 Solutions
General, Organic, and Biological Chemistry
- The correct structural for ethyne is: a. HC=CH b. HC=C=H c. HCCH d. H=C=C=Harrow_forwardAlcoholic beverages contain: a. wood alcohol. b. isopropyl alcohol. c. glyceryl alcohol. d. ethyl alcohol.arrow_forwardList the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





