
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 15, Problem 15.4QAP
Interpretation Introduction
(a)
Interpretation:
The compound which has greater fluorescence quantum yield from the following should be selected:
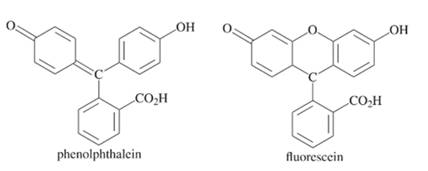
Concept introduction:
There are different cases to check the magnitude of fluorescence quantum yield of the compound. Any compound having these cases will surely have greater fluorescence quantum yield.
Interpretation Introduction
(b)
Interpretation:
The compound which has greater fluorescence quantum yield from the following should be selected:
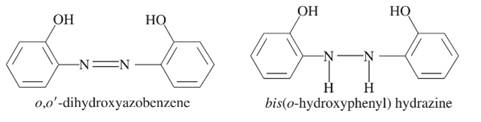
Concept introduction:
There are different scenarios to check whether the magnitude of fluorescence quantum yield of a compound is more or less. Any compound containing these scenarios will surely have more fluorescence quantum yield.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Crystal violet is a brightly colored, purple dye that can be used as a pH indicator. In the presence of a base, crystal violet reacts with OH- to become a colorless molecule. Since crystal violet is colorful violet (i.e. purple) molecule, the kinetics of this reaction can be investigated using absorbance spectroscopy as the violet color disappears. What color of light should be used for measuring the absorbance over time?
the absorbance of an equilibrium mixture of Fescn2+ was measured at 447nm and found to be 0.319. What is the equilibrium concentration of FeSCN2+?
Topic: Determination of Calcium by Atomic Absorption Spectroscopy (AAS)
Answer this: 1. It is observed that as you increase the concentration of the standard, the intensity of yellow flame also increases. Why?
Chapter 15 Solutions
Principles of Instrumental Analysis
Knowledge Booster
Similar questions
- What does the imaginary frequency observed for the transition state correspond to?arrow_forwardIf degradation occurs in a specific condition, what change in the absorbance value will be expected in the wavelength/s selected? Explain your answer.arrow_forwardDefine fluorescence spectroscopyarrow_forward
- What factors influence the wavelength of maximum emission and maximum excitation in fluorescence spectroscopy?arrow_forwardMost alkenes absorb at a shorter wavelength than alkadienes. Explain.arrow_forwardA substance has a fluorescence quantum yield of ϕF,0 = 0.16. In an experiment to measure the fluorescence lifetime of this substance, it was observed that the fluorescence emission decayed with a half-life of 1.5 ns. What is the fluorescence rate constant of this substance?arrow_forward
- When benzophenone is illuminated with ultraviolet radiation it is excited into a singlet state. Th is singlet changes rapidly into a triplet, which phosphoresces. Triethylamine acts as a quencher for the triplet. In an experi ment in methanol as solvent, the phosphorescence intensity varied with amine concentration as shown below. A timeresolved laser spectroscopy experiment had also shown that the half-life of the fluorescence in the absence of quencher is 29μs. What is the value of the quenching rate constant kO ?arrow_forwardthe d-d transition of [Ti(H2O)6]3+ produces an absorption maximum at a wavelength of about 500 nm. What is the magnitude of ∆ for [Ti(H2O)6]3+ in kJ/mol?arrow_forwardUsing the diagram below, draw arrow(s) corresponding to the process of fluorescence. Briefly explain why the emission wavelength via the fluorescence mechanism is longer than the absorbed wavelength.arrow_forward
- A solution containing a certain transition-metal complex ionhas the absorption spectrum shown here. What color would you expect a solution containing this ionto be? (a) violet (b) blue (c) green (d) orange (e) redarrow_forwardHow would you calculate Ɛ from the gradient of an absorbance vs concentration plot?arrow_forward(iii) You wish to determine the fluorescence lifetime of your sample using Time Correlated Single Photon Counting, TCSPC. Draw a sketch showing the layout and principal components of a suitable setup and explain the basis of the technique.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

