
Concept explainers
15-7 Answer true or false.
- The cis and trans stereoisomers of 2-butene are achiral.
(a)
Interpretation:
To analyse whether the given statement- The cis and trans stereoisomers of 2-butene are achiral, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
- A plane of symmetry.
- A centre of symmetry
- n-fold alternating axis of symmetry.
Answer to Problem 15.7P
The cis and trans stereoisomers of 2-butene are achiral. Thus, statement is true.
Explanation of Solution
A stereocenter is defined as an atom having groups of suitable nature so that interchange of any two groups will give a stereoisomer. However all stereocenters are not tetrahedral. The unsaturaled carbon atoms of cis-trans But-2-ene are examples of the trigonal planar stereocenter. Since an interchange of groups at these stereocenters gives a strereoisomer.
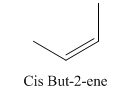 or
or 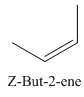
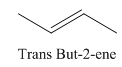 or
or 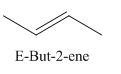
Both cis-and trans but-2-ene have non-superimposable mirror images but posses an alternating axis of symmetry, therefore cis-and trans But-2-ene are achiral.
(b)
Interpretation:
To analyse whether the given statement- The carbonyl carbon of an aldehyde, ketone, carboxylic acid, or ester cannot be a stereocenter, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
The carbonyl carbon of an aldehyde, ketone, carboxylic acid, or ester cannot be a stereocenter. Thus, statement is true.
Explanation of Solution
A stereocenter is defined as an atom having groups of suitable nature so that interchange of any two groups will give a stereoisomer. However all stereocenters are not tetrahedral. But in case of aldehyde, ketone, carboxylic acids and ester, the carbonyl carbon is sp2 hybridized due to which it forms a planar structure. Planar structures a have plane of symmetry due to which they do not possess a stereocenter. Also in carbonyl group, carbonyl carbon is linked with oxygen by two bonds that is double bond. Therefore, all the four groups are not different.
(c)
Interpretation:
To analyse whether the given statement- Stereoisomers have the same connectivity of their atoms, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
Stereoisomers have the same connectivity of their atoms. Thus, statement is true.
Explanation of Solution
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Since no bond breaking and forming takes place, thus the stereoisomers have the same connectivity of their atoms.
(d)
Interpretation:
To analyse whether the given statement- Constitutional isomers have the same connectivity of their atoms, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
Constitutional isomers do not have the same connectivity of their atoms. Thus, statement is false.
Explanation of Solution
Constitutional isomers are those isomers which have the different connectivity with atoms but have same molecular formula.
Example-
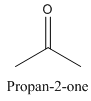 and
and 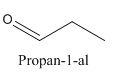
Both have same molecular formula, but they both differ in connectivity of atoms.
(e)
Interpretation:
To analyse whether the given statement-An unmarked cube is achiral, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
An unmarked cube is achiral. Thus, statement is true.
Explanation of Solution
Achiral are those compounds, that have elements of symmetry or a molecule is superimposable on its mirror image.
An unmarked cube is an achiral because it can be superimposable on its mirror image.
(f)
Interpretation:
To analyse whether the given statement-A human foot is chiral, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
A human foot is chiral. Thus, statement is true.
Explanation of Solution
If an object can be cut exactly into two equal halves so that half of its become mirror image of other half, it has plane of symmetry.
A human foot has a non-superimposable mirror image, thus human foot is chiral.
(g)
Interpretation:
To analyse whether the given statement-Every object in nature has a mirror image, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
Every object in nature has a mirror image. Thus, statement is true.
Explanation of Solution
If an object is superimposable on its mirror image, it cannot rotate plane polarized light and hence optically inactive.
If an object can be cut exactly into two equal halves so that half of its become mirror image of other half, it has plane of symmetry.
Every object in nature has a mirror image. It may or may not superimpose, and therefore the object may or may not be chiral.
(h)
Interpretation:
To analyse whether the given statement-The most common cause of chirality in organic molecules is the presence of a tetrahedral carbon atom with four different groups bonded to it, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
The most common cause of chirality in organic molecules is the presence of a tetrahedral carbon atom with four different groups bonded to it. Thus, statement is true.
Explanation of Solution
A carbon atom bonded in tetrahedral structure to four different substituents in a molecule is termed as chiral centre. It is not necessary all the time that the chiral centre is tetrahedral in shape; trigonal centres are also present in case of alkenes or unsaturated compounds.
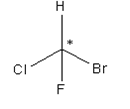
Asterisk represents the chiral centre, due to which the molecule becomes chiral.
(i)
Interpretation:
To analyse whether the given statement-If a molecule is not superposable on its mirror image, the molecule is chiral, is true or false.
Concept Introduction:
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
The elements of symmetry are-
Answer to Problem 15.7P
If a molecule is not superposable on its mirror image, the molecule is chiral. Thus, statement is true.
Explanation of Solution
A carbon atom bonded in tetrahedral structure to four different substituents in a molecule is termed as chiral centre. It is not necessary all the time that the chiral centre is tetrahedral in shape; trigonal centres are also present in case of alkenes or unsaturated compounds.
If an object is superimposable on its mirror image, it cannot rotate plane polarized light and hence optically inactive.
Want to see more full solutions like this?
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry
- 15-37 Consider a cyclohexane ring substituted with one hydroxyl group and one methyl group. Draw a structural formula for a compound of this composition that: Does not show cis-trans isomerism and has no stereocenters. Shows cis-trans isomerism but has no stereocenters. Shows cis-trans isomerism and has two stereocenters.arrow_forward15-44 Consider the structure of the immunosuppressant FK-506, a molecule shown to disrupt calcineurin- mediated signal transduction in T-lymphocytes. What is the molecular formula of this immunosuppressant? How manj' stereocenters are present in FK-506? Determine the maximum number of stereoisomers possible. Identify and label the various functional groups present. Consider the two stereocenters in this structure labeled with asterisks (*). Determine the absolute configuration of each stereocenter. FK-506 has been shown to exhibit moderate solubility in various organic solvents. Is this immunosuppressant expected to be soluble in ethanol (CH3CH2OH)? Consider the carbon atom labeled “1." Describe the geometry and approximate bond angles about this carbon atom. Draw the alternative chair conformations of the cyclohexane ring at the lower right of FK-506 and label the more stable conformation. Are there any aromatic components present in FK-506? Patients taking FK-506 have reported several side effects from this medication, including headaches, nausea or diarrhea, and slight shaking. Would you expect the enantiomer of this drug to result in the same side effects?arrow_forward15-21 Answer true or false. For a molecule with two stereocenters, 22 = 4 stereoisomers are possible. For a molecule with three stereocenters, 32 = 9 stereoisomers are possible. Enantiomers, like gloves, occur in pairs. 2-Pentanol and 3-pentanol are both chiral and show enantiomerism. 1-Methylcyclohexanol is achiral and does not show enantiomerism. Diastereomers are stereoisomers that are not mirror images.arrow_forward
- 15-26 For centuries, Chinese herbal medicine has used extracts oi Ephedra sinica to treat asthma. The asthma-relieving component of this plant is ephedrine, a very potent dilator of the air passages of the lungs. The naturally occurring stereoisomer is levorotatory and has the following structure. HO ¥ Nhch3 Ephedrine |n|„ = —41° Mark each stereocenter in epinephrine with an asterisk. How many stereoisomers are possible for this compound?arrow_forward15-16 Which of the following compounds contain stereocenters? (a) 2-Chloropentane(b) 3-Chloropentane (c) 3-Chloro-l-butene(d) 1,2-Dichloropropanearrow_forward17-54 Following is the structure of immunosuppressant FK-506, a molecule shown to disrupt calcineurin-mediated signal transduction in T-lymphocytes. (a) There are three carbon—carbon double bonds present in this molecule. Which of the three has the potential for cis/trans isomerism? Assign a cis or trans con?guration to each carbon-carbon double bond that has this possibility. (b) How many stereocenters are present in this molecule? How many stereoisomers are possible for it? (c) Are there any aromatic components in this molecule? (d) Consider the two carbon atoms marked with asterisks. Assign an R or S con?guration of each stereocenter. (e) Because of the presence of a 21-member ring, this molecule is described as a macrocycle. This ring is fashioned by three types of bonds, several carbon-carbon bonds, one ester, one hemiacetal, and one amide. Locate the ester and the hemiacetal. (f) Draw the structural formula of the long chain compound that would result if the hemiacetal were to be cleaved to an alcohol and a carbonyl group.arrow_forward
- 14-22 Arrange these compounds in order of increasing boiling point. Values in °C are —42, 78, 117, and 198 CH3CH2CH2CH2OH CH3CH2OH HOCH2CH2OH ch3ch2ch3arrow_forward15-8 What does the term “chiral” mean? Give an example of a chiral molecule.arrow_forward12-77 Show how to convert cyclopentene into these compounds. 1,2-Dibromocyclopentane Cyclopentanol Iodocyclopentane Cyclopentanearrow_forward
- 15-10 Define the term “stereoisomer.” Name three types of stereoisomers.arrow_forward16-6 Answer true or false. te/7-Butylamine is a 3° amine. In an aromatic amine, one or more of the groups bonded to nitrogen is an aromatic ring. In a heterocyclic amine, the amine nitrogen is one of the atoms of a ring. The Lewis structures of both NH4~ and CH4show the same number (eight) of valence electrons, and the VSEPR model predicts tetrahedral geometry for each. There are four constitutional isomers with the molecular formula CgH^N.arrow_forward16-17 Propylamine (bp 48°C), ethylmethylamine (bp 37°C), and trimethylamine (bp 3°C) are constitutional isomers with the molecular formula C3HgN. Account for the fact that trimethylamine has the lowest boiling point of the three and propylamine has the highest.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
