
Iodine dissolves in water, but its solubility in a nonpolar solvent such as CCl4 is greater.
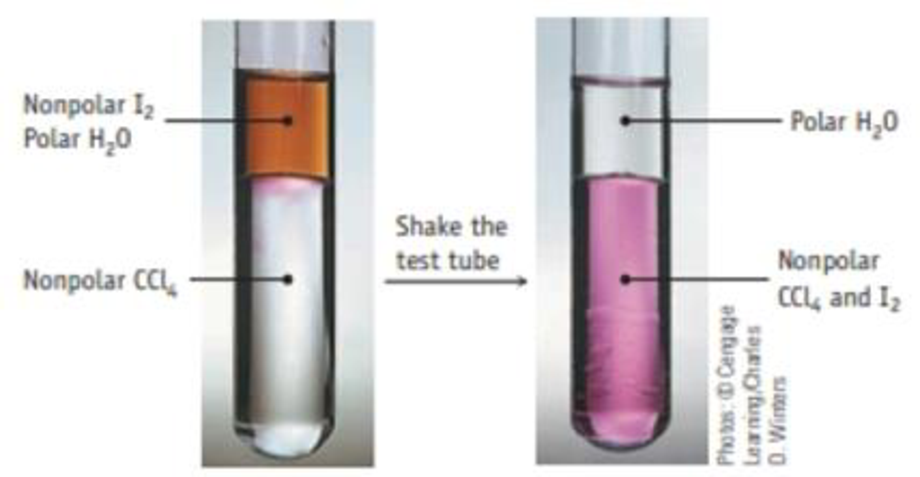
Extracting iodine (I2) from water with the nonpolar solvent CCl4. I2 is more soluble in CCl4 and, after shaking a mixture of water and CCl4, the I2 has accumulated in the more dense CCl4 layer.
The equilibrium constant is 85.0 for the process
I2(aq) ⇄ I2(CCl4)
You place 0.0340 g of I2 in 100.0 mL of water. After shaking it with 10.0 mL of CCl4, how much I2 remains in the water layer?
Trending nowThis is a popular solution!

Chapter 15 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
Inorganic Chemistry
Chemistry: Atoms First
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Chemistry: A Molecular Approach
Chemistry by OpenStax (2015-05-04)
Organic Chemistry - Standalone book
- What is the approximate value of the equilibrium constant KP for the change C2H5OC2H5(l)C2H5OC2H5(g) at 25 C. {Vapor pressure was described in the previous Chapter on liquids and solids; refer back to this chapter to find the relevant information needed to solve this problem.)arrow_forwardThe equilibrium constant for the butane iso-butane equilibrium at 25 C is 2.50. Calculate rG at this temperature in units of kJ/mol.arrow_forwardMethanol can be synthesized by means of the equilibriumreaction CO(g)+2H2(g)CH3OH(g) for which the equilibrium constant at 225°C is 6.08103. Assume that the ratio of the pressures of CO(g) and H2(g) is 1:2. What values should they have if the partial pressureof methanol is to be 0.500 atm?arrow_forward
- Which of the systems described in Exercise 13.16 give homogeneous equilibria? Which give heterogeneous equilibria?arrow_forwardDescribe a nonchemical system that is not in equilibrium, and explain why equilibrium has not been achieved.arrow_forwardWrite the expression of the reaction quotient for the ionization of HOCN in water.arrow_forward
- The atmosphere consists of about 80% N2 and 20% O2, yet there are many oxides of nitrogen that are stable and can be isolated in the laboratory. (a) Is the atmosphere at chemical equilibrium with respect to forming NO? (b) If not, why doesnt NO form? If so, how is it that NO can be made and kept in the laboratory for long periods?arrow_forwardIf wet silver carbonate is dried in a stream of hot air. the air must have a certain concentration level of carbon dioxide to prevent silver carbonate from decomposing by the reaction Ag2CO3(s)Ag2O(s)+CO2(g) H for this reaction is 79.14 kJ/mol in the temperature range of 25 to 125C. Given that the partial pressure of carbon dioxide in equilibrium with pure solid silver carbonate is 6.23 103 torr at 25C, calculate the partial pressure of CO2 necessary to prevent decomposition ofAg2CO3 at 110C. (Hint: Manipulate the equation in Exercise 79.)arrow_forwardKc = 5.6 1012 at 500 K for the dissociation of iodine molecules to iodine atoms. I2(g) 2 I(g) A mixture has [I2] = 0.020 mol/Land [I] = 2.0 108 mol/L. Is the reaction at equilibrium (at 500 K)? If not, which way must the reaction proceed to reach equilibrium?arrow_forward
- In a solution with carbon tetrachloride as the solvent, the compound VCl4. undergoes dimerization: 2VCl4V2Cl8 When 6.6834 g VCl4. is dissolved in 100.0 g carbon tetrachloride, the freezing point is lowered by 5.97C. Calculate the value of the equilibrium constant for the dimerization of VCl4 at this temperature. (The density of the equilibrium mixture is 1.696 g/cm3, and Kf = 29.8C kg/mol for CCl4.)arrow_forwardFor the system 2SO3(g)2SO2(g)+O2(g) K=1.32 at 627. What is the equilibrium constant at 555C?arrow_forwardBecause calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go a step further and examine the equilibrium between carbonate ion and CaCOj. The reaction is Ca2+(aq) + COj2_(aq) ** CaCO,(s) The equilibrium constant for this reaction is 2.1 X 10*. If the initial calcium ion concentration is 0.02 AI and the carbonate concentration is 0.03 AI, what are the equilibrium concentrations of the ions? A student is simulating the carbonic acid—hydrogen carbonate equilibrium in a lake: H2COj(aq) H+(aq) + HCO}‘(aq) K = 4.4 X 10"7 She starts with 0.1000 AI carbonic acid. What are the concentrations of all species at equilibrium?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





