
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15, Problem 3SC
Interpretation Introduction
Interpretation:
The structures of ethyl-2-methylbutanoate and civetone are to be examined and upon opening a bottle containing the mixture of these two, which one would dominate initial smell is to be determined.
Concept introduction:
Civetone is an unsaturated macrocyclic
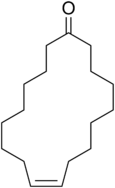
Civetone
Graham’s law states that the rate of diffusion is inversely proportional to the square root of mass of its particles.
Molar mass is defined as the ratio of moles
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Aldehydes have which type of smell?
Fish like smell
Pungent smell
Bitter almond smell
Rotten egg like smell
Explain why NOT ALL hydrolysis of esters produce a sour smell
Hello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 15 minutes, NO NEED TO SHOW THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you.
What class of organic chemical compounds is responsible for the fragrant smell of several fruits?
Alcohols
Esters
Amines
Alkanes
Thiols
Chapter 15 Solutions
Chemistry In Focus
Ch. 15 - Prob. 15.1YTCh. 15 - Prob. 1SCCh. 15 - Prob. 2SCCh. 15 - Prob. 3SCCh. 15 - Prob. 1ECh. 15 - Prob. 2ECh. 15 - Prob. 3ECh. 15 - Prob. 4ECh. 15 - Prob. 5ECh. 15 - Prob. 6E
Ch. 15 - Prob. 7ECh. 15 - Prob. 8ECh. 15 - Prob. 9ECh. 15 - Prob. 10ECh. 15 - Prob. 11ECh. 15 - Prob. 12ECh. 15 - Prob. 13ECh. 15 - Define eutrophication.Ch. 15 - Prob. 15ECh. 15 - Prob. 16ECh. 15 - Prob. 17ECh. 15 - Prob. 18ECh. 15 - Prob. 19ECh. 15 - What are the three types of interactions that...Ch. 15 - Prob. 21ECh. 15 - Prob. 22ECh. 15 - Prob. 23ECh. 15 - Prob. 24ECh. 15 - Prob. 25ECh. 15 - Prob. 26ECh. 15 - Prob. 27ECh. 15 - Prob. 28ECh. 15 - Prob. 29ECh. 15 - How do sunscreens protect your skin from the Suns...Ch. 15 - Prob. 31ECh. 15 - Prob. 32ECh. 15 - Prob. 33ECh. 15 - Prob. 34ECh. 15 - Prob. 35ECh. 15 - Prob. 36ECh. 15 - Prob. 37ECh. 15 - Prob. 38ECh. 15 - Prob. 39ECh. 15 - Prob. 40ECh. 15 - Prob. 41ECh. 15 - Prob. 42ECh. 15 - Prob. 43ECh. 15 - Prob. 44ECh. 15 - Prob. 45ECh. 15 - Prob. 46ECh. 15 - The salt bridges that hold hair protein (keratin)...Ch. 15 - Prob. 48ECh. 15 - The hydrochloric acid present in toilet bowl...Ch. 15 - Prob. 50ECh. 15 - Prob. 51ECh. 15 - Prob. 52ECh. 15 - Prob. 53ECh. 15 - Prob. 54ECh. 15 - Prob. 55ECh. 15 - Prob. 56ECh. 15 - Prob. 57ECh. 15 - Prob. 58ECh. 15 - Prob. 59ECh. 15 - Prob. 60ECh. 15 - Prob. 61ECh. 15 - Prob. 62ECh. 15 - Prob. 64ECh. 15 - Prob. 65E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Organic chemistry:How do you account for the smell of vinegar when an old bottle of aspirin is opened ?arrow_forwardCompare the processes that occur when methanol (CH3OH), hydrogen chloride (HCl), and sodium hydroxide (NaOH) dissolve in water.arrow_forwardAa.87. What are the flavor compound categories and primary characteristics? a. Nonvolatile (does not easily vaporize) b. Volatile (aroma's, can typically be smelled as these easily go into gas phase)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co