
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 96AE
One method for determining the purity of aspirin (C9H8O4) is to hydrolyze it with NaOH solution and then to titrate the remaining NaOH. The reaction of aspirin with NaOH is as follows:
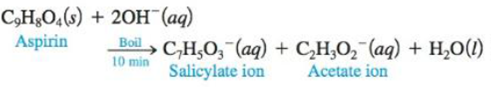
A sample of aspirin with a mass of 1.427 g was boiled in 50.00 mL of 0.500 M NaOH. After the solution was cooled, it took 31.92 mL of 0.289 M HCl to titrate the excess NaOH. Calculate the purity of the aspirin. What indicator should be used for this titration? Why?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 15 Solutions
Chemistry
Ch. 15 - What is meant by the presence of a common ion? How...Ch. 15 - Define a buffer solution. What makes up a buffer...Ch. 15 - One of the most challenging parts of solving...Ch. 15 - A good buffer generally contains relatively equal...Ch. 15 - Draw the general titration curve for a strong acid...Ch. 15 - Instead of the titration of a strong acid by a...Ch. 15 - Sketch the titration curve for a weak acid...Ch. 15 - Sketch the titration curve for a weak base...Ch. 15 - What is an acidbase indicator? Define the...Ch. 15 - Why does an indicator change from its acid color...
Ch. 15 - What are the major species in solution after...Ch. 15 - A friend asks the following: Consider a buffered...Ch. 15 - Mixing together solutions of acetic acid and...Ch. 15 - Could a buffered solution be made by mixing...Ch. 15 - Sketch two pH curves, one for the titration of a...Ch. 15 - Sketch a pH curve for the titration of a weak acid...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - The common ion effect for weak acids is to...Ch. 15 - Prob. 10QCh. 15 - A best buffer has about equal quantities of weak...Ch. 15 - Consider the following pH curves for 100.0 mL of...Ch. 15 - An acid is titrated with NaOH. The following...Ch. 15 - Consider the following four titrations. i. 100.0...Ch. 15 - Figure 14-4 shows the pH curves for the titrations...Ch. 15 - Acidbase indicators mark the end point of...Ch. 15 - How many of the following are buffered solutions?...Ch. 15 - Which of the following can be classified as buffer...Ch. 15 - A certain buffer is made by dissolving NaHCO3 and...Ch. 15 - A buffer is prepared by dissolving HONH2 and...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Compare the percent dissociation of the acid in...Ch. 15 - Compare the percent ionization of the base in...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Which of the solutions in Exercise 21 shows the...Ch. 15 - Prob. 30ECh. 15 - Calculate the pH of a solution that is 1.00 M HNO2...Ch. 15 - Calculate the pH of a solution that is 0.60 M HF...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of a buffered solution prepared...Ch. 15 - A buffered solution is made by adding 50.0 g NH4Cl...Ch. 15 - Calculate the pH after 0.010 mole of gaseous HCl...Ch. 15 - An aqueous solution contains dissolved C6H5NH3Cl...Ch. 15 - Calculate the mass of sodium acetate that must be...Ch. 15 - What volumes of 0.50 M HNO2 and 0.50 M NaNO2 must...Ch. 15 - Consider a solution that contains both C5H5N and...Ch. 15 - Calculate the ratio [NH3]/[NH4+] in...Ch. 15 - Carbonate buffers are important in regulating the...Ch. 15 - When a person exercises, muscle contractions...Ch. 15 - Consider the acids in Table 13-2. Which acid would...Ch. 15 - Consider the bases in Table 13-3. Which base would...Ch. 15 - Calculate the pH of a solution that is 0.40 M...Ch. 15 - Calculate the pH of a solution that is 0.20 M HOCl...Ch. 15 - Which of the following mixtures would result in...Ch. 15 - Which of the following mixtures would result in a...Ch. 15 - What quantity (moles) of NaOH must be added to 1.0...Ch. 15 - Calculate the number of moles of HCl(g) that must...Ch. 15 - Consider the titration of a generic weak acid HA...Ch. 15 - Sketch the titration curve for the titration of a...Ch. 15 - Consider the titration of 40.0 mL of 0.200 M HClO4...Ch. 15 - Consider the titration of 80.0 mL of 0.100 M...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M...Ch. 15 - Lactic acid is a common by-product of cellular...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Calculate the pH at the halfway point and at the...Ch. 15 - In the titration of 50.0 mL of 1.0 M methylamine,...Ch. 15 - You have 75.0 mL of 0.10 M HA. After adding 30.0...Ch. 15 - A student dissolves 0.0100 mole of an unknown weak...Ch. 15 - Two drops of indicator HIn (Ka = 1.0 109), where...Ch. 15 - Methyl red has the following structure: It...Ch. 15 - Potassium hydrogen phthalate, known as KHP (molar...Ch. 15 - A certain indicator HIn has a pKa of 3.00 and a...Ch. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 74ECh. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 76ECh. 15 - Estimate the pH of a solution in which bromcresol...Ch. 15 - Estimate the pH of a solution in which crystal...Ch. 15 - A solution has a pH of 7.0. What would be the...Ch. 15 - A solution has a pH of 4.5. What would be the...Ch. 15 - Derive an equation analogous to the...Ch. 15 - a. Calculate the pH of a buffered solution that is...Ch. 15 - Tris(hydroxymethyl)aminomethane, commonly called...Ch. 15 - You make 1.00 L of a buffered solution (pH = 4.00)...Ch. 15 - You have the following reagents on hand: Solids...Ch. 15 - Prob. 86AECh. 15 - Phosphate buffers are important in regulating the...Ch. 15 - What quantity (moles) of HCl(g) must be added to...Ch. 15 - Prob. 89AECh. 15 - The following plot shows the pH curves for the...Ch. 15 - Calculate the volume of 1.50 102 M NaOH that must...Ch. 15 - Prob. 92AECh. 15 - A certain acetic acid solution has pH = 2.68....Ch. 15 - A 0.210-g sample of an acid (molar mass = 192...Ch. 15 - The active ingredient in aspirin is...Ch. 15 - One method for determining the purity of aspirin...Ch. 15 - A student intends to titrate a solution of a weak...Ch. 15 - A student titrates an unknown weak acid, HA, to a...Ch. 15 - A sample of a certain monoprotic weak acid was...Ch. 15 - Consider 1.0 L of a solution that is 0.85 M HOC6H5...Ch. 15 - What concentration of NH4Cl is necessary to buffer...Ch. 15 - Consider the following acids and bases: HCO2H Ka =...Ch. 15 - Consider a buffered solution containing CH3NH3Cl...Ch. 15 - Consider the titration of 150.0 mL of 0.100 M HI...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M HCN...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the following four titrations (iiv): i....Ch. 15 - Another way to treat data from a pH titration is...Ch. 15 - A buffer is made using 45.0 mL of 0.750 M HC3H5O2...Ch. 15 - A 0.400-M solution of ammonia was titrated with...Ch. 15 - What volume of 0.0100 M NaOH must be added to 1.00...Ch. 15 - Consider a solution formed by mixing 50.0 mL of...Ch. 15 - When a diprotic acid. H2A. is titrated with NaOH,...Ch. 15 - Prob. 114CPCh. 15 - The titration of Na2CO3 with HCl bas the following...Ch. 15 - Consider the titration curve in Exercise 115 for...Ch. 15 - A few drops of each of the indicators shown in the...Ch. 15 - Malonic acid (HO2CCH2CO2H) is a diprotic acid. In...Ch. 15 - A buffer solution is prepared by mixing 75.0 mL of...Ch. 15 - A 10.00-g sample of the ionic compound NaA, where...Ch. 15 - Calculate the pH of a solution prepared by mixing...Ch. 15 - Consider a solution prepared by mixing the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Consider a sample of ideal gas initially in a volume V at temperature T and pressure P. Does the entropy of thi...
General Chemistry: Principles and Modern Applications (11th Edition)
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 10. L of water is added to 3.0 L of 6.0 M H2SO4, what is the molarity of the resulting solution? Assume the volumes are additive.arrow_forwardA 25.0-mL sample of sodium sulfate solution was analyzed by adding an excess of barium chloride solution to produce barium sulfate crystals, which were filtered from the solution. Na2SO4(aq)+BaCl2(aq)2NaCl(aq)+BaSO4(s) If 5.719 g of barium sulfate was obtained, what was the molarity of the original Na2SO4 solution?arrow_forwardA 1.345-g sample of a compound of barium and oxygen was dissolved in hydrochloric acid to give a solution of barium ion, which was then precipitated with an excess of potassium chromate to give 2.012 g of barium chromate, BaCrO4. What is the formula of the compound?arrow_forward
- You want to prepare a 1.0 mol/kg solution of ethyleneglycol, C2H4(OH)2, in water. Calculate the mass of ethylene glycol you would need to mix with 950. g water.arrow_forwardSodium chloride is used in intravenous solutions for medical applications. The NaCl concentration in such solutions must be accurately known and can be assessed by reacting the solution with an experimentally determined volume of AgNO3 solution of known concentration. The net ionic equation is Ag+(aq)+Cl(aq)AgCl(s) Suppose that a chemical technician uses 19.3 mL of 0.200-M AgNO3 to convert all the NaCl in a 25.0-mL sample of an intravenous solution to AgCl. Calculate the molarity of NaCl in the solution.arrow_forwardTwenty-five mL of a 0.388 M solution of Na2SO4 is mixed with 35.3 mL of 0.229 M Na2SO4. What is the molarity of the resulting solution? Assume that the volumes are additive.arrow_forward
- A 10.00-mL sample of a 24.00% solution of ammonium bromide (NH4Br) requires 23.41 mL of 1.200 molar silver nitrate (AgNO3) to react with all of the bromide ion present. (a) Calculate the molarity of the ammonium bromide solution. (b) Use the molarity of the solution to find the mass of ammonium bromide in 1.000 L of this solution. (c) From the percentage concentration and the answer to part b, find the mass of 1.000 L ammonium bromide solution. (d) Combine the answer to part c with the volume of 1.000 L to express the density of the ammonium bromide solution (in g/mL).arrow_forward39. Standard solutions of calcium ion used to test for water hardness are prepared by dissolving pure calcium carbonate. CaCO3, in dilute hydrochloric acid. A 1.745-g sample of CaCO3 is placed in a 250.O-mL volumetric flask and dissolved in HCI. Then the solution is diluted to the calibration mark of the volumetric flask. Calculate the resulting molarity of calcium ion.arrow_forwardOn Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The spill was neutralized with sodium carbonate: 2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+H2O(l)+CO2(g) a. Calculate H for this reaction. Approximately 2.0 104 gal nitric acid was spilled. Assume that the acid was an aqueous solution containing 70.0% HNO3 by mass with a density of 1.42 glcm3. What mass of sodium carbonate was required for complete neutralization of the spill, and what quantity of heat was evolved? (Hf for NaNO3(aq) = 467 kJ/mol) b. According to The Denver Post for April 4, 1983, authorities feared that dangerous air pollution might occur during the neutralization. Considering the magnitude of H, what was their major concern?arrow_forward
- Bone was dissolved in hydrochloric acid, giving 50.0 mL of solution containing calcium chloride, CaCL2. To precipitate the calcium ion from the resulting solution, an excess of potassium oxalate was added. The precipitate of calcium oxalate, CaC2O4, weighed 1.437 g. What was the molarity of CaCl2 in the solution?arrow_forwardCitric acid, which can be obtained from lemon juice, has the molecular formula C6H8O7. A 0.250-g sample of citric acid dissolved in 25.0 mL of water requires 37.2 mL of 0.105 M NaOH for complete neutralization. What number of acidic hydrogens per molecule does citric acid have?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Thermogravimetric Analysis [ TGA ] # Thermal Analysis # Analytical Chemistry Part-11# CSIR NET/GATE; Author: Priyanka Jain;https://www.youtube.com/watch?v=p1K-Jpzylso;License: Standard YouTube License, CC-BY