
(a)
Interpretation:
To identify the
Concept introduction:
The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
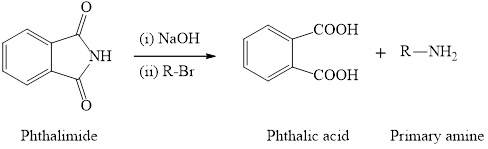
(b)
Interpretation: To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction: The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(c)
Interpretation:
To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction:
The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(d)
Interpretation:
To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction: The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry (8th Edition)
- Three of the following amines can be prepared by the Gabriel synthesis; three cannot. Write equations showing the successful applications of this method. (a) Butylamine (b) Isobutylamine (c) tert-Butylamine (d) 2-Phenylethylamine (e) N-Methylbenzylamine (f) Anilinearrow_forwardSynthesis of p-Bromoaniline Why is the protection of the amine function needed in this reaction? a) The protection increases the selectivity for the ortho substitution. b) The protection increases the reactivity of the reactant in the bromination. c) The protection changes the regioselectivity of reaxtion.arrow_forwardPreamble: A student chemist in an attempt to synthesise compound B from the aromatic aldehyde. 24. What is the reaction name for the chemical transformation of A to BA. reductive aminationB. catalytic reductionC. carbonyl dehydrationD. Hofmann eliminationE. Aldehyde rearrangementarrow_forward
- give the results of the following compounds after reaction with nitrous acid test a. primary aliphatic amine b. secondary aliphatic amine c. tertiary aliphatic amine d. primary aromatic aminearrow_forwardwhat is the role of triethylamine in this reaction? Briefly desrcibe how ninhydrin works.arrow_forwardDraw the component of the opioid analgesic structure which is consistent between morphine, oxycodone and codeine (backbone of the opioid analgesics).arrow_forward
- please help with this question. thank you. n-acetylazoles go through hydrolysis more than regular amides. propose a reason for the reactivity of n-acetylazoles toward nucleophilic substitution.arrow_forwardPredict the chemical name of compound B a. p-chlorobenzylamine b. 4-bromoaniline c. benzylamine d. p-chloronitrile e. p-chlorobenzaldehyde 2. What is the reaction name for the chemical transformation of A to B a. reductive amination b. catalytic reduction c. carbonyl dehydration d. Hofmann elimination e. Aldehyde rearrangementarrow_forwardWhy do you wash the dichloromethane solution of your reductive amination product with sodium bicarbonate, rather than dilute aqueous HCl? a) Sodium bicarbonate is a good method of removing aldehydes from organic solvent.b) The amine product will be protonated by acid and remain in the aqueous layer as a salt.c) Sodium bicarbonate transfers the amine starting material into the aqueous layer.d) Sodium bicarbonate reacts with leftover NaBH(OAc)3 and removes it from the mixture.arrow_forward
- A. The water solubility of amines found in medications can be increased by salt formation. Dextromethorphan hydrobromide, a cough suppresant, is the salt formed from dextromethorphan (XX) and hydrobromic acid (HBr). Write the equation for the formation of dextromethorphan hydrobromide. B. Suggest appropriate starting materials ( amine and alkyl halide) needed for the preparation of each of the following salts: 1. Cetylpyridinium chloride 2. Benzyldimethyltetradecylammonium chloridearrow_forwardWhat is the most likely organic product of the exhaustive hydrolysis of PhCN? A. benzoic acid B. benzamide C. benzylamine D. benzenearrow_forward1. Why does H2 not give an IR spectrum? 2. Explain why primary amines and unsubstituted amides have two NH stretching absorptions. 3. Why do anhydrides show two carbonyl peaks? 4. HCl is known to give addition reactions to carbon-carbon double bonds. Why is this behavior not observed in this reaction? 5. Predict the structure of the product expected from addition of molecular bromine to maleic acid.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

