
a)
Interpretation:
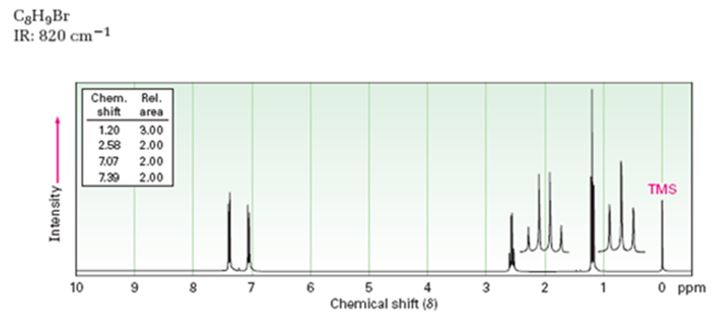
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C8H9Br I.R: 820 cm-1.
1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet).
Concept introduction:
In 1HNMR spectrum
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1 and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C8H9Br I.R: 820 cm-1.
1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet).
Answer to Problem 47AP
A structure for the compound with M.F = C8H9Br with spectral characteristics: I.R: 820 cm-1, 1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet) is
Explanation of Solution
The molecular formula of the compound is C8H9Br.
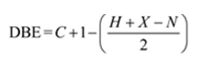
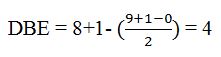
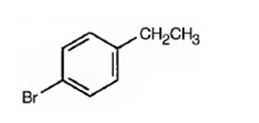
Thus the compound has four unsaturation units like double bonds and/or rings. The two doublets at 7.07δ and 7.39δ each corresponding to two protons indicate the compound is aromatic and each proton responsible for the doublet has a proton on the adjacent carbon. The two proton quartet at 2.58δ (benzylic) and three proton triplet at 1.20δ (primary) could be accounted for only if an ethyl substituent is present on the ring along with bromine. The I.R absorption at 820 cm-1 indicates that the ethyl group and bromine are arranged in para positions. Hence the compound is p-bromoethyl benzene.
A structure for the compound with M.F=C8H9Br with spectral characteristics: I.R: 820 cm-1, 1HNMR spectrum: 7.07δ (2H, doublet); 7.39δ (2H, doublet); 2.58δ (2H, quartet); 1.20δ (3H, triplet) is

b)
Interpretation:
A structure for the compound whose 1HNMR spectrum given is to be proposed.
Given: M.F = C9H12 I.R: 750 cm-1
1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet).
Concept introduction:
In 1HNMR spectrum aromatic protons give a broad peak in the range 6.5δ-8.0δ, the primary alkyl protons around 0.7δ-1.3δ, the secondary alkyl protons around 1.2δ-1.6δ, and a tertiary alkyl protons in between 1.4δ-1.8δ. The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12 I.R: 750 cm-1.
1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet).
Answer to Problem 47AP
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12, I.R: 750 cm-1, 1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet) is
Explanation of Solution
The molecular formula of the compound is C9H12.
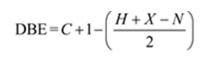
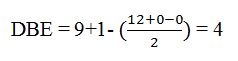
Thus the compound has four unsaturation units like double bonds and/or rings. The four proton broad band at 7.13δ indicates that the compound is aromatic with two substituent groups attached to the benzene ring. The two proton quartet at 2.64δ (benzylic) and three proton triplet at 1.19δ (primary alkyl) could be accounted for only if an ethyl group is present on the ring. The three proton singlet at 2.31δ (benzylic) can be attributed to a methyl group attached to the ring. Thus the two substituent groups attached to the benzene ring are ethyl and methyl. The I.R absorption at 750 cm-1 requires that the ethyl and methyl groups to be arranged in ortho positions. Hence the compound is o-ethyltoluene.
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C9H12, I.R: 750 cm-1, 1HNMR spectrum: 7.13δ (4H, broad); 2.64δ (2H, quartet); 2.31δ (3H, singlet); 1.19δ (3H, triplet) is

c)
Interpretation:
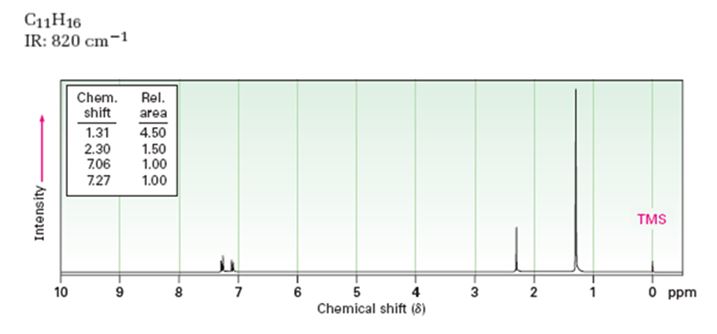
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16 I.R: 820 cm-1.
1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Concept introduction:
In 1HNMR spectrum aromatic protons give a broad peak in the range 6.5δ-8.0δ, the primary alkyl protons around 0.7δ-1.3 δ, the secondary alkyl protons around 1.2δ-1.6δ, and a tertiary alkyl protons in between 1.4δ-1.8δ. The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In I.R, the o-disubstituted benzenes absorb around 735-770 cm-1, m-disubstituted benzenes absorb around 690-710 cm-1 and p-disubstituted benzenes absorb around 810-840 cm-1.
To propose:
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16 I.R: 820 cm-1.
1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Answer to Problem 47AP
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16; I.R: 820 cm-1; 1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).
Explanation of Solution
The molecular formula of the compound is C11H16.
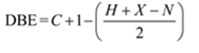
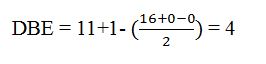
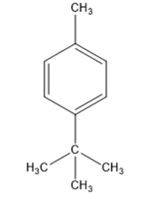
Thus the compound has four unsaturation units like double bonds and/or rings. The two doublets at 7.27δ and 7.06δ each corresponding to two protons indicate the compound is aromatic and each proton responsible for the doublet has a proton on the adjacent carbon. The three proton singlet at 2.58δ (benzylic) could be accounted for a methyl group attached to the benzene ring. The remaining four carbons and nine hydrogens indicate the presence of a tert-butyl group attached to the benzene ring which is confirmed by the nine proton singlet at 1.31δ (primary alkyl). Thus methyl and tert-butyl are the two substituent groups on the benzene ring. The I.R absorption at 820 cm-1 indicates that the two substituent groups are arranged in para positions. Hence the compound is p-t-butyltoluene.
A structure for the compound whose 1HNMR spectrum given is to be proposed. Given: M.F = C11H16; I.R: 820 cm-1; 1HNMR spectrum: 7.27δ (2H, doublet); 7.06δ (2H, doublet); 2.30δ (3H, singlet); 1.31δ (9H, singlet).

Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
- Propose a structure for each of the following two isomers with formula C6H14 given their 1H-NMR spectra. Isomer A: δ = 0.84 (d, 12 H), 1.39 (septet, 2H) ppm Isomer B: δ = 0.84 (t, 3 H), 0.86 (t, 9H), 1.22 (q, 2H) ppmarrow_forwardCompound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound. Please show work.arrow_forwardFollowing are 1H-NMR spectra for compounds B (C6H12O2) and C (C6H10O). Upon warming in dilute acid, compound B is converted to compound C. Deduce the structural formulas for compounds B and C.arrow_forward
- A compound of formula C6H10O2 shows only two absorptions in the proton NMR: a singlet at 2.67 ppm and a singlet at2.15 ppm. These absorptions have areas in the ratio 2:3. The IR spectrum shows a strong absorption at 1708 cm-1. Proposea structure for this compound.arrow_forward1, 6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at -0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.arrow_forwardPropose structures for alcohols that have the following 1HNMR spectra: (a) C5H12Oarrow_forward
- Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forwardThe 1H and 13C NMR spectra below belong to a compound with formula C6H10O2. Propose a structure for this compound.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forward
- Compound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 î disappears when D2O is added.arrow_forwardPropose a structure for compound X (molecular formula C6H12O2), which gives a strong peak in its IR spectrum at 1740 cm−1. The 1H NMR spectrum of X shows only two singlets, including one at 3.5 ppm. The 13C NMR spectrum is given below. Propose a structure for X.arrow_forwardThe 1H-NMR spectrum of Compound D of molecular formula C10H12O shows three singlets – δ 2.20 (6H, s), 4.86 (4H), 7.10 (2H) ppm. Its 13C-NMR spectrum has five signals – 20, 74, 127, 135, 146 ppm. Suggest a structure for this compound.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

