
Concept explainers
Draw the structure consistent with each description.
a.
b.
c.
(a)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The prefix
Answer to Problem 16.10P
The structure corresponding to
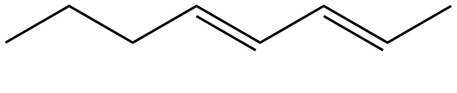
Explanation of Solution
The given name is
Thus, the correct structure of
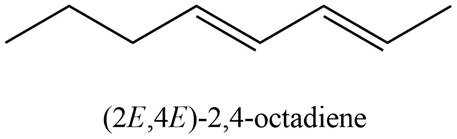
Figure 1
The structure corresponding to
(b)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The prefix
Answer to Problem 16.10P
The structure corresponding to
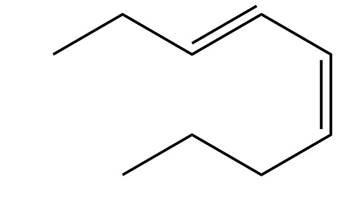
Explanation of Solution
The given name is
Thus, the correct structure of
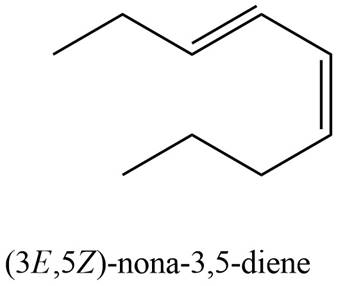
Figure 2
The structure corresponding to
(c)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The prefix
Answer to Problem 16.10P
The structures corresponding to
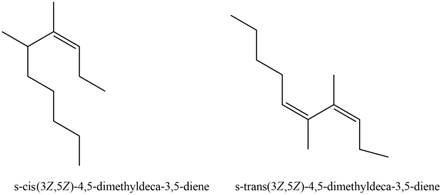
Explanation of Solution
The given name is
Thus, the correct structure to
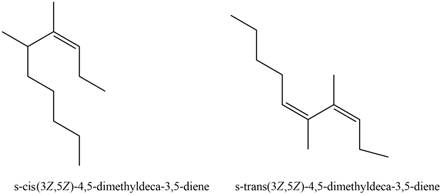
Figure 3
The structure corresponding to
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- 1, part A) We observe 6 carbon stereocenters in the molecule below. Indicate each stereocenter, and give the absolute S or R configuration. 1, part B) Indicate each stereoisomer in this molecule, and give the absolute S or R configuration. Then, indicate E or Z configuration of all alkenes in it (*ignoring aromatic ring*).arrow_forwardWhich group in each pair is assigned the higher priority in R,S nomenclature? a. – CD3, – CH3 b. – CH(CH3)2, – CHOH c. – CH2Cl, – CH2CH2CH2Br d. – CH2NH2, – NHCH3arrow_forwardBuild a model of methylcyclohexane, and use the model to complete the following Newmanprojections of methylcyclohexane in the chair conformation: a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation. b. Label one Newman projection above anti and the other gauche to describe the relationshipbetween the methyl group and C3 of the ring. c. In general, which is a lower PE conformation, anti or gauche? d. Explain how your answer to b and c provide an explanation for why it is more favorable fora large group to be in an equatorial than an axial position.arrow_forward
- Draw the structure of each compound.a. (Z)-penta-1,3-diene in the s-trans conformationb. (2E,4Z)-1-bromo-3-methylhexa-2,4-dienec. (2E,4E,6E)-octa-2,4,6-triened. (2E,4E)-3-methylhexa-2,4-diene in the s-cis conformationarrow_forwardWhich group in each pair is assigned the higher priority in R,S nomenclature? a.−CD3, −CH3 b.−CH(CH3)2, −CH2OH c.−CH2Cl, −CH2CH2CH2Br d.−CH2NH2, −NHCH3arrow_forwardOrder the following substituents from highest priority to lowest priority: CH3, OH, H,F. Use the Cahn-Ingold-Prelog priority system. a. F, CH3, OH, H b. H, CH3, F, OH c. F, OH, CH3, H d. H, CH3, OH, Farrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
