
Concept explainers
a Draw a pH titration curve that represents the titration of 50.0 mL of 0.10 M NH3 by the addition of 0.10 M HCl from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 30%, 50%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?
(a)
Interpretation:
For titration of 50.0 mL of 0.10 M
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 30 %, 50% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.119QP
A pH titration curve showing the equivalence point and buffer region is given in Figure 1 as follows,
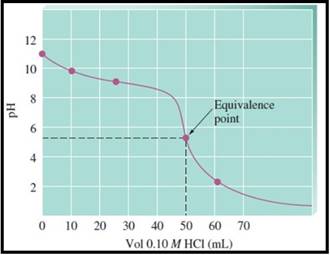
Figure 1
(a)
The pH at the 0% titration point is 11.13
The pH at the 30% titration point is 9.62
The pH at the 50% titration point is 9.26
The pH at the 100% titration point is 5.28
(b)
The solution at the equivalence point is acidic
Explanation of Solution
To Calculate: The pH of the titration points for the 0%, 30 %, 50% and 100%
Given data:
Titration of 0.10 M
pH at the 0% titration point:
Construct an equilibrium table with x as unknown concentration
|
|
|||
| Initial |
0.10
0.10-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Substitute equilibrium concentrations into the equilibrium-constant equation.
The
Assume x is negligible compared to 0.10 M
Therefore, the concentration of hydroxide ion
In the end, pH is calculated as follows,
Therefore, the pH at the 0% titration point is 11.13
pH at the 30% titration point:
For convenience, express the concentrations as percents.
Substitute the concentrations into the equilibrium expression.
Therefore, the concentration of hydroxide ion
In the end, pH is calculated as follows,
Therefore, the pH at the 30% titration point is 9.62
pH at the 50% titration point:
The pH is calculated as follows,
Therefore, the pH at the 50% titration point is 9.26
pH at the 100% titration point:
As a result of titration
The
Therefore,
Construct an equilibrium table with x as unknown concentration
|
|
|||
| Initial |
0.0500
0.0500-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Now, calculate
Substitute into the equilibrium constant expression.
Here, x gives the concentration of hydronium ion,
The pH is calculated as follows,
Therefore, the pH at the 100% titration point is 5.28
The pH at the 0% titration point was calculated as 11.13
The pH at the 30% titration point was calculated as 9.62
The pH at the 50% titration point was calculated as 9.26
The pH at the 100% titration point was calculated as 5.28
(b)
Interpretation:
For titration of 50.0 mL of 0.10 M
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 30 %, 50% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.119QP
A pH titration curve showing the equivalence point and buffer region is given in Figure 1 as follows,
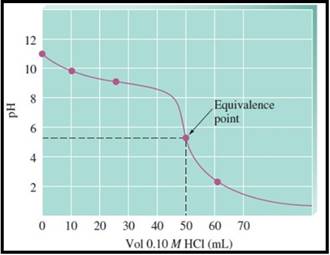
Figure 1
(a)
The pH at the 0% titration point is 11.13
The pH at the 30% titration point is 9.62
The pH at the 50% titration point is 9.26
The pH at the 100% titration point is 5.28
(b)
The solution at the equivalence point is acidic
Explanation of Solution
To Explain: Whether the solution at the equivalence point is neutral, acidic or basic
As a result of titration
Ammonium chloride salt is the salt of weak base and a strong acid
Therefore, the given solution is acidic
The solution at the equivalence point was found as acidic
Want to see more full solutions like this?
Chapter 16 Solutions
General Chemistry - Standalone book (MindTap Course List)
- For the titration of 50.0 mL of 0.150 M ethylamine. C2H5NH2, with 0.100 M HCl, find the pH at each of the following points, and then use that information to sketch the titration curve and decide on an appropriate indicator. (a) At the beginning, before HCl is added (b) At the halfway point in the titration (c) When 75% of the required acid has been added (d) At the equivalence point (e) When 10.0 mL more HCl has been added than is required (f) Sketch the titration curve. (g) Suggest an appropriate indicator for this titration.arrow_forwardThe titration curves for two acids with the same base are shown on this graph. (a) Which is the curve for the weaker acid? Explain your choice. (b) Give the approximate pH at the equivalence point for the titration of each acid. (c) Explain why the pH at the equivalence point differs for each acid. (d) Explain why the starting pH values of the two acids differ. (e) Which indicator or indicators, phenolphthalein, bromthymol blue, or methyl red, could be used for the titration of Acid 1? For the titration of Acid 2? Explain your choices.arrow_forwarda Draw a pH titration curve that represents the titration of 25.0 mL of 0.15 M propionic acid. CH3CH2COOH, by the addition of 0.15 M KOH from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 50%, 60%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?arrow_forward
- A 0.2481 M solution of KOH is used to titrate 30.00 mL of 0.269 M hydrobromic acid. Assume that volumes are additive. (a) Write a balanced net ionic equation for the reaction that takes place during the titration. (b) What are the species present at the equivalence point? (c) What volume of KOH is required to reach the equivalence point? (d) What is the pH of the solution 1. before any KOH is added? 2. halfway to the equivalence point? 3. at the equivalence point?arrow_forwardChloropropionic acid, ClCH2CH2COOH, is a weak monoprotic acid with Ka = 7.94 105. Calculate the pH at the equivalence point in a titration of 10.00 mL of 0.100 M chloropropionic acid with 0.100 M KOH. Choose an indicator from Table 16.4 for the titration. Explain your choice. TABLE 16.5 Properties of Several Indicatorsarrow_forwardFor the titration of 50.0 mL of 0.100-M HCl with 0.100-M NaOH, calculate the pH when these volumes of NaOH have been added: (a) 10.0 mL (b) 25.00 mL (c) 45.0 mL (d) 50.5 mLarrow_forward
- When 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence point is reached when 35.00 mL base has been added. After 20.00 mL NaOH solution has been added, the titration mixture has a pH of 5.75. Calculate the ionization constant of the acid.arrow_forwardA solution is prepared by dissolving 0.350 g of benzoic acid, HC7H5O2, in water to make 100.0 mL of solution. A 30.00-mL sample of the solution is titrated with 0.272 M KOH. Calculate the pH of the solution (a) before titration. (b) halfway to the equivalence point. (c) at the equivalence point.arrow_forwardCalculate the pH during the titration of 50.00 mL of 0.100 M Sr(OH)2 with 0.100 M HNO3 after 0, 50.00, 100.00, and 150.00 mL nitric acid have been added. Graph the titration curve and compare with the titration curve obtained in Exercise 16.22.arrow_forward
- If added to 1 L of 0.20-M NaOH, which of these would form a buffer? (a) 0.10 mol acetic acid (b) 0.30 mol acetic acid (c) 0.20 mol HCl (d) 0.10 mol NaCH3COO Explain your answers.arrow_forwardRepeat the procedure in Exercise 61, but for the titration of 25.0 mL of 0.100 M pyridine with 0.100 M hydrochloric acid (Kb for pyridine is 1.7 109). Do not calculate the points at 24.9 and 25.1 mL.arrow_forwardFifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a) before any NaOH is added? (b) at half-neutralization? (c) at the equivalence point? (d) when 0.10 mL less than the volume of NaOH to reach the equivalence point is added? (e) when 0.10 mL more than the volume of NaOH to reach the equivalence point is added? (f) Use your data to construct a plot similar to that shown in Figure 14.10 (pH versus volume NaOH added).arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





