
(a)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
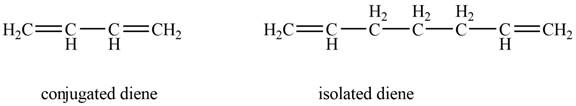
Figure 1
Answer to Problem 16.1P
The given diene is a conjugated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
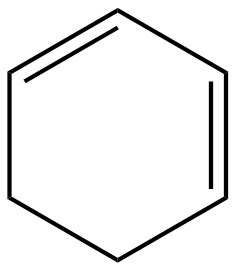
Figure 2
In the given diene, two double bonds are separated by a single bond. Thus, the given diene is a conjugated diene.
The given diene is a conjugated diene.
(b)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
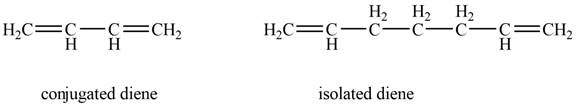
Figure 1
Answer to Problem 16.1P
The given diene is an isolated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
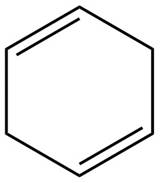
Figure 3
In the given diene, two double bonds are separated by a two sigma bonds. Thus, the given diene is an isolated diene.
The given diene is an isolated diene.
(c)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
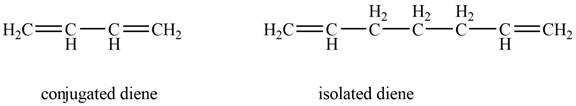
Figure 1
Answer to Problem 16.1P
The given diene is a conjugated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
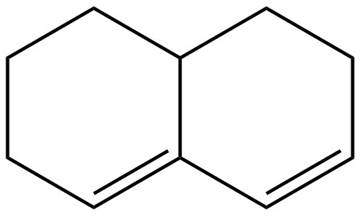
Figure 4
In the given diene, two double bonds are separated by a single bond. Thus, the given diene is a conjugated diene.
The given diene is a conjugated diene.
(d)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated idene is shown below.
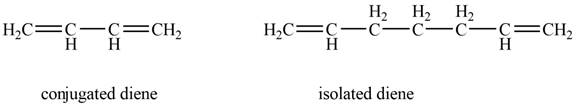
Figure 1
Answer to Problem 16.1P
The given diene is an isolated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
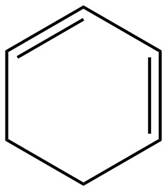
Figure 5
In the given diene, two double bonds are separated by more than single bond. Thus, the given diene is an isolated diene.
The given diene is an isolated diene.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry - Access (Custom)
- Addition of HCl to alkene X forms two alkyl halides Y and Z. (A) Label Y and Z as a kinetic or thermodynamic product and explain why. (B) Explain why the addition of HCl occurs at the exocyclic C=C, rather than the other C=Carrow_forwardWhich is the major product?a. Ab. Neither product would likely formc. Barrow_forwardRank the attached alkenes in order of increasing stability.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
