
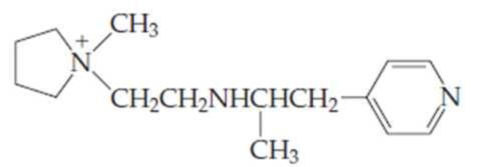
- (a) For the compound above, identify each nitrogen as either a primary, secondary, tertiary, quaternary, or aromatic
amine . - (b) Which amine group(s) would be able to provide a hydrogen bond? Which could accept a hydrogen bond?
(a)
Interpretation:
It should be identified that the each nitrogen in the given compound either as a primary, secondary, tertiary amine, or aromatic amine.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
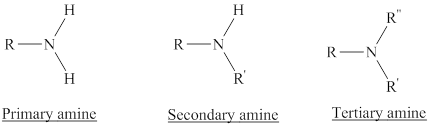
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Example: Tetramethylammonium ion
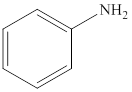
Aniline is an aromatic amine compound and its structure is,
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
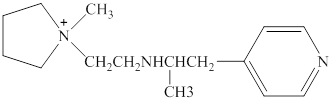
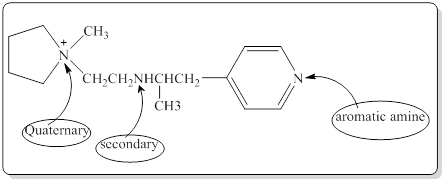
As per the concepts above mentioned,
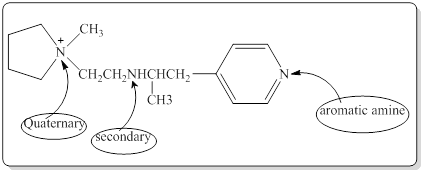
Each nitrogen atoms in the given compound can be identified either as a primary, secondary, tertiary amine or aromatic amine as follows,
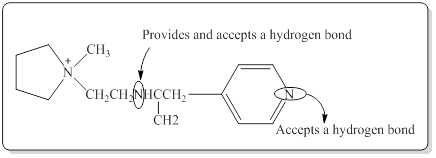
(b)
Interpretation:
The amine group which would be able to provide a hydrogen bond and accept a hydrogen bond in the given compound has to be identified.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Hydrogen bond is an attractive force established between hydrogen atom attached to a highly electronegative element and another highly electronegative element of the same or different molecule.
Answer to Problem 16.23UKC
Explanation of Solution
Structure of the given compound is,
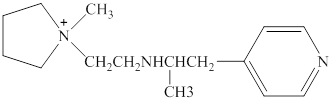
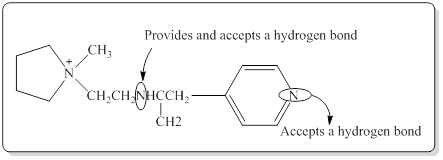
In a hydrogen bond strong partial positive charge on hydrogen attracts lone electron pair on oxygen or nitrogen.
Here in the given molecule, an amine group which was able to provide a hydrogen bond and accept a hydrogen bond and those amines can be identified as follows,
Want to see more full solutions like this?
Chapter 16 Solutions
Fund. of General, Org... -Masteringchem.
- Why are disulfide bonds particularly important and unique in their role as determinants of tertiary structure?arrow_forwardwhat determines if a bond is polar?arrow_forwardWhen two atoms share a pair of valence electrons with different levels of electronegativity, what type of bond is this?arrow_forward
- How does the bonding involved in a compound (nanoscopic interactions) influence the macroscopic physical properties that can be observed of the compound?arrow_forwardGive the molecular formula of the functional group that is missing. a. NH+ b. CH3 c. COOH d. C6H12O6arrow_forwardIdentify the component monosaccharides of each of the following compounds and describe the type of glycosidic linkage in each.arrow_forward
- Which of the functional groups listed in shown Table can function as hydrogen bond donors? As hydrogen bond acceptors?arrow_forwardFor A, B, C, D, E, F, identify the circled functional groups and linkages in the compound in the picture.arrow_forwardWhy is a thioester bond a “high-energy” bond?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





