
Concept explainers
(a)
Interpretation:
The complete equation for the reaction between an acid chloride and ammonia is to be stated.
Concept introduction:
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
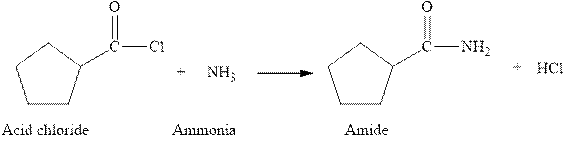
The complete reaction between an acid chloride and ammonia is given below.
Explanation of Solution
The reaction between an acid chloride and ammonia gives amide and hydrochloric acid. This happens by the attack of lone pair of electrons of nitrogen in ammonia on the carbonyl carbon of acid chloride. The intermediate then rearranges to give amide and hydrochloric acid.
The complete reaction between an acid chloride and ammonia is given below.

Figure 1
The complete reaction between an acid chloride and ammonia is shown in Figure 1.
(b)
Interpretation:
The complete equation for the reaction between an amine and
Concept introduction:
Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
The complete reaction between an amine and carboxylic acid is given below.
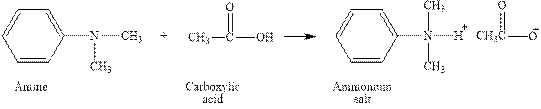
Explanation of Solution
The reaction between an amine and carboxylic acid gives ammonium salt of carboxylic acid. This happens by the abstraction of proton by lone pair of electrons of nitrogen in amine from the carboxylic acid. The intermediate then rearranges to give ammonium salt of carboxylic acid.
The complete reaction between an amine and carboxylic acid is given below.
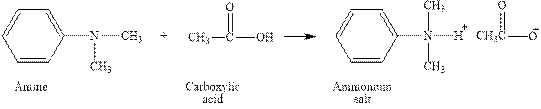
Figure 2
The complete reaction between an amine and carboxylic acid is shown in Figure 2.
(c)
Interpretation:
The complete equation for the reaction between a tertiary amine and acid chloride is to be stated.
Concept introduction:
Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
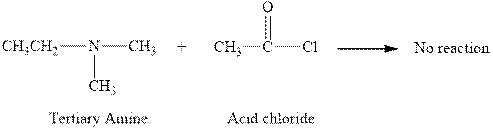
The reaction between a tertiary amine and acid chloride does not occur and this is shown below.
Explanation of Solution
The reaction between a tertiary amine and acid chloride does not occur. This happens due to the absence of hydrogen atom in the tertiary amine. The complete reaction between a tertiary amine and acid chloride can be written as given below.

Figure 3
The reaction between a tertiary amine and acid chloride does not occur and this is shown in Figure 3.
(d)
Interpretation:
The complete equation for the reaction between a secondary amine and hydrochloric acid is to be stated.
Concept introduction:
Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
The complete reaction between a secondary amine and hydrochloric acid is given below.

Explanation of Solution
The reaction between a secondary amine and hydrochloric acid gives salt of amine. This happens by the abstraction of proton by lone pair of electrons of nitrogen in amine from the hydrochloric acid. The intermediate then rearranges to give salt of amine.
The complete reaction between a secondary amine and hydrochloric acid is given below.

Figure 4
The complete reaction between a secondary amine and hydrochloric acid is shown in Figure 4.
(e)
Interpretation:
The complete equation for the reaction between a secondary amine and water is to be stated.
Concept introduction:
Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
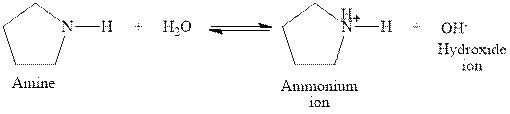
The complete reaction between a secondary amine and water is given below.
Explanation of Solution
The reaction between a secondary amine and water gives ammonium ion and hydroxide ion. This happens by the abstraction of proton by lone pair of electrons of nitrogen in amine from the water. The intermediate then rearranges to give ammonium ion and hydroxide ion.
The complete reaction between a secondary amine and water is given below.

Figure 5
The complete reaction between a secondary amine and water is shown in Figure 5.
(f)
Interpretation:
The complete equation for the reaction between an amine salt and sodium hydroxide is to be stated.
Concept introduction:
Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 16.25E
The complete reaction between an amine salt and sodium hydroxide is given below.
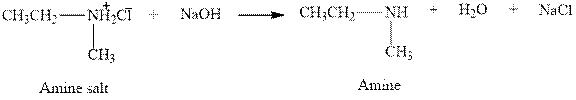
Explanation of Solution
The reaction between an amine salt and sodium hydroxide gives amine, water and sodium chloride. This happens by the abstraction of proton by lone pair of electrons of oxygen in sodium hydroxide from the amine salt. The intermediate then rearranges to give amine, water and sodium chloride.
The complete reaction between an amine salt and sodium hydroxide is given below.
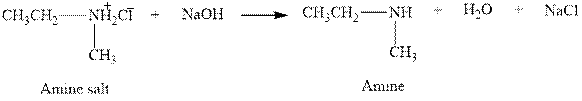
Figure 6
The complete reaction between an amine salt and sodium hydroxide is shown in Figure 6.
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Give a clear explanation handwritten answer...explain each point...arrow_forwardcan you draw a organic molecule following these criteria: contains a hydroxyl group. would produce a carboxylic acid upon reaction with KMnO4. contains at least two branches. is represented in a condensed formula diagramarrow_forwardPlease explain and give names of A and Barrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

