
Concept explainers
(a)
Interpretation:
Procaine was one of the first local anesthetics. Its hydrochloride salt is marketed as Novocain.
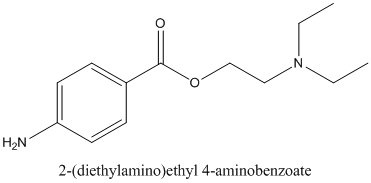
If procaine is chiral or not and if it contains any stereocenter should be identified.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
(b)
Interpretation:
The nitrogen atom of procaine which is a stronger base should be identified.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
(c)
Interpretation:
A structural formula for the salt formed by treating procaine with one mole of HCl, showing which nitrogen is protonated and bears the positive charge should be determined.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- Calculate the pKa of each acid and indicate which is the stronger acid. C6O2H8, Ka = 1.7 x 10–5 C2O4H2, Ka = 5.9 x 10–2arrow_forwardGiven that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the water layer?arrow_forwardFor an acid with a pKa of 5.00, what is the pKb of its conjugate base?arrow_forward
- please find Ka values of Succinic Acid (C4H6O4)arrow_forwardThe side chain of cysteine is weakly acidic. Suppose in a protein, the side chain of a cysteine residue is surrounded by the side chains of several isoleucine residues. Would this make the side chain of the cysteine residue more acidic or less acidic? Please explain your answer.arrow_forwardUsing the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forward
- A chemically modified guanidino group is present in cimetidine (Tagamet), a widely prescribed drug for the control of gastric acidity and peptic ulcers. Cimetidine reduces gastric acid secretion by inhibiting the interaction of histamine with gastric H2 receptors. In the development of this drug, a cyano group was added to the substituted guanidino group to alter its basicity. Do you expect this modified guanidino group to be more basic or less basic than the guanidino group of arginine? Explain.arrow_forwardCalculate the pKa of a acid at 25°C if its conjugate base has a pKb = 1.19arrow_forwardDraw the structure of the conjugate base that will be formed when 1 mole of HOCH2CO2H reacts with 1 mole of CH3CH2CH2Li.arrow_forward
- Will CH3CH2NH2 act as a Bronsted-Lowry acid or base when reacting with water?arrow_forwardDuring acidosis of a protein, shown below, salt bridges are broken, resulting in denaturation. Which choice shows the products of alkalosis?arrow_forwardFor the following molecule, draw the major species at each pH. The carboxylic acid is drawn as a conjugate acid and the amine are drawn as the conjugate base. 1. pH of 2 2. pH of 4 3. pH of 7arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



