
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
14th Edition
ISBN: 9781259327933
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.79QP
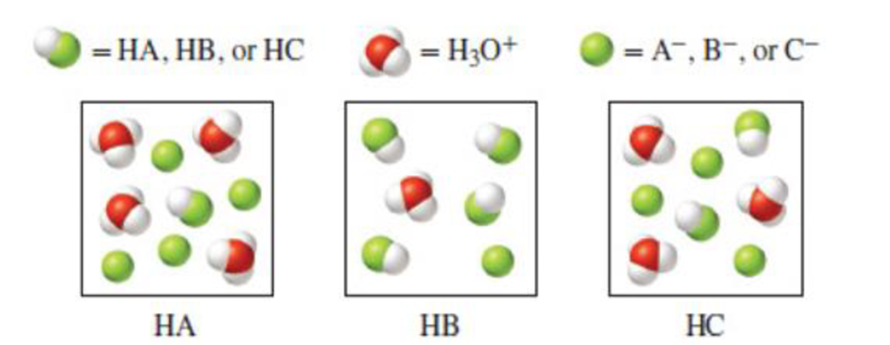
The following diagrams represent aqueous solutions of three different monoprotic acids: HA, HB, and HC. (a) Which conjugate base (A−, B−, or C−) has the smallest Kb value? (b) Which anion is the strongest base? The water molecules have been omitted for clarity.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
Ch. 16.1 - What is (a) the conjugate base of HNO3, (b) the...Ch. 16.1 - What is (a) the conjugate acid of ClO4, (b) the...Ch. 16.1 - HSO3 is the conjugate acid of what species? HSO3...Ch. 16.1 - Which of the models represents a species that has...Ch. 16.1 - Prob. 16.2WECh. 16.1 - Identify and label the species in each reaction....Ch. 16.1 - Prob. 2PPBCh. 16.1 - Write the formula and charge for each species in...Ch. 16.1 - Prob. 16.1.1SRCh. 16.1 - Prob. 16.1.2SR
Ch. 16.2 - Predict the relative strengths of the oxoacids in...Ch. 16.2 - Prob. 3PPACh. 16.2 - Based on the information in this section, which is...Ch. 16.2 - Prob. 3PPCCh. 16.2 - Prob. 16.2.1SRCh. 16.2 - Prob. 16.2.2SRCh. 16.2 - Prob. 16.2.3SRCh. 16.3 - Prob. 16.4WECh. 16.3 - The concentration of hydroxide ions in the antacid...Ch. 16.3 - The value of Kw at normal body temperature (37C)...Ch. 16.3 - Prob. 4PPCCh. 16.3 - Prob. 16.3.1SRCh. 16.3 - Prob. 16.3.2SRCh. 16.4 - Determine the pOH of a solution at 25C in which...Ch. 16.4 - Determine the pOH of a solution at 25C in which...Ch. 16.4 - Determine the pOH of a solution at 25C in which...Ch. 16.4 - Prob. 5PPCCh. 16.4 - Calculate the hydroxide ion concentration in a...Ch. 16.4 - Prob. 6PPACh. 16.4 - Prob. 6PPBCh. 16.4 - Prob. 6PPCCh. 16.4 - Prob. 16.4.1SRCh. 16.4 - Prob. 16.4.2SRCh. 16.4 - Prob. 16.4.3SRCh. 16.4 - Prob. 16.4.4SRCh. 16.5 - Calculate the pH of an aqueous solution at 25C...Ch. 16.5 - Prob. 7PPACh. 16.5 - Prob. 7PPBCh. 16.5 - Prob. 7PPCCh. 16.5 - Prob. 16.8WECh. 16.5 - Calculate the concentration of HNO3 in a solution...Ch. 16.5 - Prob. 8PPBCh. 16.5 - Which of the plots [(i)(iv)] best approximates the...Ch. 16.5 - Prob. 16.9WECh. 16.5 - Prob. 9PPACh. 16.5 - Prob. 9PPBCh. 16.5 - Prob. 9PPCCh. 16.5 - Prob. 16.10WECh. 16.5 - Prob. 10PPACh. 16.5 - Prob. 10PPBCh. 16.5 - Prob. 10PPCCh. 16.5 - Prob. 16.5.1SRCh. 16.5 - Prob. 16.5.2SRCh. 16.5 - Prob. 16.5.3SRCh. 16.5 - Prob. 16.5.4SRCh. 16.5 - Prob. 16.5.5SRCh. 16.5 - Prob. 16.5.6SRCh. 16.5 - Prob. 16.5.7SRCh. 16.6 - The Ka of hypochlorous acid (HClO) is 3.5 108....Ch. 16.6 - Calculate the pH at 25C of a 0.18-M solution of a...Ch. 16.6 - Prob. 11PPBCh. 16.6 - The diagrams show solutions of four different weak...Ch. 16.6 - Determine the pH and percent ionization for acetic...Ch. 16.6 - Determine the pH and percent ionization for...Ch. 16.6 - At what concentration does hydrocyanic acid...Ch. 16.6 - Prob. 12PPCCh. 16.6 - Aspirin (acetylsalicylie acid, HC9H7O4) is a weak...Ch. 16.6 - Prob. 13PPACh. 16.6 - Prob. 13PPBCh. 16.6 - Calculate Ka values (to two significant figures)...Ch. 16.6 - Prob. 16.6.1SRCh. 16.6 - Prob. 16.6.2SRCh. 16.6 - Prob. 16.6.3SRCh. 16.7 - Prob. 16.14WECh. 16.7 - Calculate the pH at 25C of a 0.0028-M solution of...Ch. 16.7 - Prob. 14PPBCh. 16.7 - The diagrams represent solutions of three...Ch. 16.7 - Caffeine, the stimulant in coffee and tea, is a...Ch. 16.7 - Prob. 15PPACh. 16.7 - Prob. 15PPBCh. 16.7 - Prob. 15PPCCh. 16.7 - Prob. 16.7.1SRCh. 16.7 - Prob. 16.7.2SRCh. 16.7 - Prob. 16.7.3SRCh. 16.8 - Prob. 16.16WECh. 16.8 - Prob. 16PPACh. 16.8 - Prob. 16PPBCh. 16.8 - Prob. 16PPCCh. 16.8 - Prob. 16.8.1SRCh. 16.8 - Prob. 16.8.2SRCh. 16.8 - Prob. 16.8.3SRCh. 16.9 - Oxalic acid (H2C2O4) is a poisonous substance used...Ch. 16.9 - Calculate the concentrations of H2C2O4, HC2O4,...Ch. 16.9 - Calculate the concentrations of H2SO4, HSO4, SO42,...Ch. 16.9 - Prob. 17PPCCh. 16.9 - Prob. 16.9.1SRCh. 16.9 - Prob. 16.9.2SRCh. 16.9 - Prob. 16.9.3SRCh. 16.10 - Prob. 16.18WECh. 16.10 - Determine the pH of a 0.15-M solution of sodium...Ch. 16.10 - Prob. 18PPBCh. 16.10 - Winch of the graphs [(i)(iv)] best represents the...Ch. 16.10 - Calculate the pH of a 0.10-M solution of ammonium...Ch. 16.10 - Determine the pH of a 0.25-M solution of...Ch. 16.10 - Prob. 19PPBCh. 16.10 - Prob. 19PPCCh. 16.10 - Predict whether a 0.10-M solution of each of the...Ch. 16.10 - Predict whether a 0.10-M solution of each of the...Ch. 16.10 - Prob. 20PPBCh. 16.10 - Prob. 20PPCCh. 16.10 - Prob. 16.10.1SRCh. 16.10 - Prob. 16.10.2SRCh. 16.10 - Prob. 16.10.3SRCh. 16.10 - Prob. 16.10.4SRCh. 16.10 - Prob. 16.10.5SRCh. 16.12 - Identify the Lewis acid and Lewis base in each of...Ch. 16.12 - Prob. 21PPACh. 16.12 - Prob. 21PPBCh. 16.12 - Which of the diagrams best depicts the combination...Ch. 16.12 - Prob. 16.12.1SRCh. 16.12 - Prob. 16.12.2SRCh. 16 - F or a species to act as a Brnsted base, an atom...Ch. 16 - Identify the acid-base conjugate pairs in each of...Ch. 16 - Prob. 16.3QPCh. 16 - Prob. 16.4QPCh. 16 - Write the formulas of the conjugate bases of the...Ch. 16 - Prob. 16.6QPCh. 16 - Prob. 16.7QPCh. 16 - List four factors that affect the strength of an...Ch. 16 - Prob. 16.9QPCh. 16 - Prob. 16.10QPCh. 16 - Prob. 16.11QPCh. 16 - Prob. 16.12QPCh. 16 - Prob. 16.13QPCh. 16 - Write the equilibrium expression for the...Ch. 16 - Write an equation relating [H+] and [OH] in...Ch. 16 - Write an equation relating [H+] and [OH] in...Ch. 16 - Prob. 16.17QPCh. 16 - Prob. 16.18QPCh. 16 - Prob. 16.19QPCh. 16 - Prob. 16.20QPCh. 16 - Prob. 16.21QPCh. 16 - Prob. 16.22QPCh. 16 - Prob. 16.23QPCh. 16 - Calculate the concentration of H+ ions in a 0.62 M...Ch. 16 - Calculate the concentration of OH ions in a 1.4 ...Ch. 16 - Calculate the pH of each of the following...Ch. 16 - Calculate the pH of each of the following...Ch. 16 - Prob. 16.28QPCh. 16 - Prob. 16.29QPCh. 16 - Prob. 16.30QPCh. 16 - How much NaOH (in grams) is needed to prepare 546...Ch. 16 - Prob. 16.32QPCh. 16 - Why are ionizations of strong acids and strong...Ch. 16 - Prob. 16.34QPCh. 16 - Prob. 16.35QPCh. 16 - Prob. 16.36QPCh. 16 - Prob. 16.37QPCh. 16 - Prob. 16.38QPCh. 16 - Prob. 16.39QPCh. 16 - Prob. 16.40QPCh. 16 - Prob. 16.41QPCh. 16 - Prob. 16.42QPCh. 16 - Prob. 16.43QPCh. 16 - Prob. 16.1VCCh. 16 - Prob. 16.2VCCh. 16 - Prob. 16.3VCCh. 16 - Prob. 16.4VCCh. 16 - Prob. 16.44QPCh. 16 - Prob. 16.45QPCh. 16 - Prob. 16.46QPCh. 16 - Why do we normally not quote Ka values for strong...Ch. 16 - Why is it necessary to specify temperature when...Ch. 16 - Which of the following solutions has the highest...Ch. 16 - Prob. 16.50QPCh. 16 - The Ka for benzoic acid is 6.5 105. Calculate the...Ch. 16 - Calculate the pH of an aqueous solution at 25C...Ch. 16 - Calculate the pH of an aqueous solution at 25C...Ch. 16 - Determine the percent ionization of the following...Ch. 16 - Determine the percent ionization of the following...Ch. 16 - Prob. 16.56QPCh. 16 - A 0.015-M solution of a monoprotic acid is 0.92%...Ch. 16 - Prob. 16.58QPCh. 16 - Prob. 16.59QPCh. 16 - Prob. 16.60QPCh. 16 - Prob. 16.61QPCh. 16 - Prob. 16.62QPCh. 16 - In biological and medical applications, it is...Ch. 16 - Classify each of the following species as a weak...Ch. 16 - Prob. 16.65QPCh. 16 - Prob. 16.66QPCh. 16 - Prob. 16.67QPCh. 16 - Which of the following has a higher pH: (a) 1.0 M...Ch. 16 - Prob. 16.69QPCh. 16 - Prob. 16.70QPCh. 16 - Prob. 16.71QPCh. 16 - What is the original molarity of an aqueous...Ch. 16 - Prob. 16.73QPCh. 16 - Prob. 16.74QPCh. 16 - Prob. 16.75QPCh. 16 - Prob. 16.76QPCh. 16 - Prob. 16.77QPCh. 16 - Calculate Ka for each of the following ions: NH4+,...Ch. 16 - The following diagrams represent aqueous solutions...Ch. 16 - Prob. 16.80QPCh. 16 - Write all the species (except water) that are...Ch. 16 - Write the Ka1 and Ka2 expressions for sulfurous...Ch. 16 - Prob. 16.83QPCh. 16 - Prob. 16.84QPCh. 16 - Prob. 16.85QPCh. 16 - Prob. 16.86QPCh. 16 - Calculate the pH at 25C of a 0.25-M aqueous...Ch. 16 - The first and second ionization constants of a...Ch. 16 - Prob. 16.89QPCh. 16 - Prob. 16.90QPCh. 16 - Explain why small, highly charged metal ions are...Ch. 16 - Prob. 16.92QPCh. 16 - Specify which of the following salts will undergo...Ch. 16 - Prob. 16.94QPCh. 16 - Calculate the pH of a 0.42 M NH4Cl solution. (Kb...Ch. 16 - Calculate the pH of a 0.082 M NaF solution. (Ka...Ch. 16 - Calculate the pH of a 0.91 M C2H5NH3I solution....Ch. 16 - Prob. 16.98QPCh. 16 - Predict whether the following solutions are...Ch. 16 - Prob. 16.100QPCh. 16 - In a certain experiment, a student finds that the...Ch. 16 - Prob. 16.102QPCh. 16 - Prob. 16.103QPCh. 16 - Classify the following oxides as acidic, basic,...Ch. 16 - Prob. 16.105QPCh. 16 - Explain why metal oxides tend to be basic if the...Ch. 16 - Arrange the oxides in each of the following groups...Ch. 16 - Prob. 16.108QPCh. 16 - Prob. 16.109QPCh. 16 - Prob. 16.110QPCh. 16 - Prob. 16.111QPCh. 16 - Prob. 16.112QPCh. 16 - In terms of orbitals and electron arrangements,...Ch. 16 - Prob. 16.114QPCh. 16 - Prob. 16.115QPCh. 16 - Which would be considered a stronger Lewis acid:...Ch. 16 - Prob. 16.117QPCh. 16 - Identify the Lewis acid and the Lewis base in the...Ch. 16 - Identify the Lewis acid and the Lewis base in the...Ch. 16 - Prob. 16.120QPCh. 16 - Prob. 16.121QPCh. 16 - Prob. 16.122QPCh. 16 - Prob. 16.123QPCh. 16 - Prob. 16.124QPCh. 16 - Calculate the pH and percent ionization of a 0.88...Ch. 16 - Prob. 16.126QPCh. 16 - Prob. 16.127QPCh. 16 - The pH of a 0.0642-M solution of a monoprotic acid...Ch. 16 - Prob. 16.129QPCh. 16 - HA and HB are both weak acids although HB is the...Ch. 16 - Prob. 16.131QPCh. 16 - Prob. 16.132QPCh. 16 - Use the data in Table 16.5 to calculate the...Ch. 16 - Prob. 16.134QPCh. 16 - Most of the hydrides of Group 1A and Group 2 A...Ch. 16 - Prob. 16.136QPCh. 16 - Novocaine, used as a local anesthetic by dentists,...Ch. 16 - Which of the following is the stronger base: NF3...Ch. 16 - Prob. 16.139QPCh. 16 - The ion product of D20 is 1.35 1015 at 25C. (a)...Ch. 16 - Prob. 16.141QPCh. 16 - Prob. 16.142QPCh. 16 - Prob. 16.143QPCh. 16 - Prob. 16.144QPCh. 16 - Prob. 16.145QPCh. 16 - When the concentration of a strong acid is not...Ch. 16 - Calculate the pH of a 2.00 M NH4CN solution.Ch. 16 - Prob. 16.148QPCh. 16 - Prob. 16.149QPCh. 16 - Prob. 16.150QPCh. 16 - Prob. 16.151QPCh. 16 - Hydrocyanic acid (HCN) is a weak acid and a deadly...Ch. 16 - How many grams of NaCN would you need to dissolve...Ch. 16 - Prob. 16.154QPCh. 16 - Calculate the pH of a 1-L solution containing...Ch. 16 - Prob. 16.156QPCh. 16 - You are given two beakers, one containing an...Ch. 16 - Use Le Chteliers principle to predict the effect...Ch. 16 - A 0.400 M formic acid (HCOOH) solution freezes at...Ch. 16 - The disagreeable odor of fish is mainly due to...Ch. 16 - Prob. 16.161QPCh. 16 - Prob. 16.162QPCh. 16 - Both the amide ion (NH2) and the nitride ion (N3)...Ch. 16 - When carbon dioxide is bubbled through a clear...Ch. 16 - Explain the action of smelling salt, which is...Ch. 16 - About half of the hydrochloric acid produced...Ch. 16 - Which of the following does not represent a Lewis...Ch. 16 - Determine whether each of the following statements...Ch. 16 - How many milliliters of a strong monoprotic acid...Ch. 16 - Hemoglobin (Hb) is a blood protein that is...Ch. 16 - Prob. 16.171QPCh. 16 - Calculate the pH of a solution that is 1.00 M HCN...Ch. 16 - Tooth enamel is largely hydroxyapatite...Ch. 16 - Prob. 16.174QPCh. 16 - Prob. 16.175QPCh. 16 - Prob. 16.176QPCh. 16 - Sulfuric acid (H2SO4) accounts for as much as 80...Ch. 16 - A 1-87-g sample of Mg reacts with 80.0 mL of a HCl...Ch. 16 - Calculate the pH of a solution that is 0.22 M in...Ch. 16 - Determine pH at the equivalence point in the...Ch. 16 - Calculate the pH of a solution that is 0.22 M in...Ch. 16 - Determine pH at the equivalence point in the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the freezing point of vinegar, which is an aqueous solution of 5.00% acetic acid, HC2H3O2, by mass (d=1.006g/cm3)?arrow_forwardUsing the diagrams shown in Problem 10-37, which of the four acids is the weakest acid?arrow_forwardWrite the reaction and the corresponding Kb equilibrium expression for each of the following substances acting as bases in water. a. aniline, C6H5NH2 b. dimethylamine, (CH3)2NHarrow_forward
- Aluminum chloride, AlCl3, reacts with trimethyl-amine, N(CH3)3. What would you guess to be the product of this reaction? Explain why you think so. Describe the reaction in terms of one of the acid base concepts. Write an appropriate equation to go with this description. Which substance is the acid according to this acidbase concept? Explain.arrow_forwardUsing the diagrams shown in Problem 10-117, which of the solutions would have the greatest buffer capacity, that is, greatest protection against pH change, when the following occurs? a. A strong acid is added to the solution. b. A strong base is added to the solution.arrow_forwardFor oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forward
- 12.62 Write the formula of the conjugate acid of each of the following bases, (a) OH-, (b) NHj, (c) CHjNHt, (d) HPO/-, (e) CO.,2’arrow_forwardPure liquid ammonia ionizes in a manner similar to that of water. (a) Write the equilibrium for the autoionization of liquid ammonia. (b) Identify the conjugate acid form and the base form of the solvent. (c) Is NaNH2 an acid or a base in this solvent? (d) Is ammonium bromide an acid or a base in this solvent?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY