
Concept explainers
(a)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given species and its hybrid are,
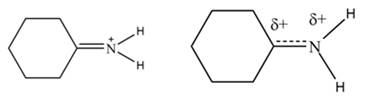
The increasing order of stability for the given resonance structure is,
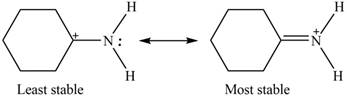
Explanation of Solution
The given species is shown below.
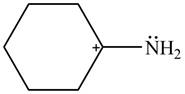
Figure 1
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
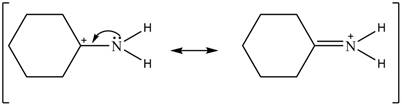
Figure 2
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
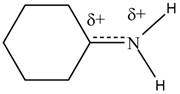
Figure 3
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
The Figure 2 shows that the left side structure does not contain double bond. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.
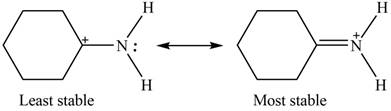
Figure 4
The second resonance structure for given species and its hybrid is shown in Figure 2 and 3. The increasing order of stability for the given resonance structure is shown in Figure 4.
(b)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given species and its hybrid are,
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
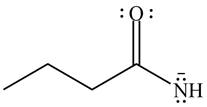
Figure 5
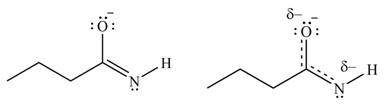
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
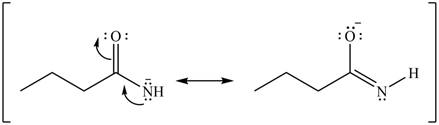
Figure 6
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
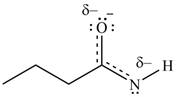
Figure 7
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure in which negative charge is on more electronegative atom. The electronegativity of oxygen is more than nitrogen.
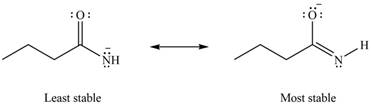
The Figure 6 shows that in the left side structure, negative charge is on nitrogen atom, whereas in right side structure, negative charge is on oxygen atom.. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.
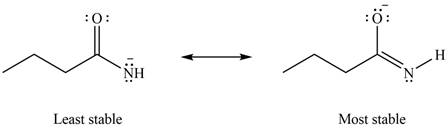
Figure 8
The second resonance structure for given species and its hybrid is shown in Figure 6 and 7. The increasing order of stability for the given resonance structure is shown in Figure 8.
(c)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given carbocation and its hybrid are,
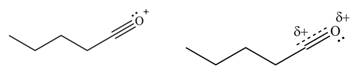
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
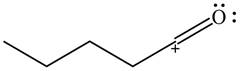
Figure 9
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
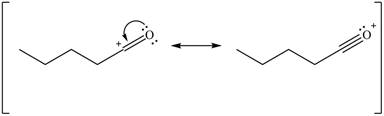
Figure 10
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
Figure 11
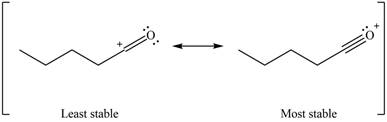
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
Therefore, the increasing order of stability for the given resonance structure is shown below.

Figure 12
The second resonance structure for given carbocation and its hybrid is shown in Figure 10 and 11. The increasing order of stability for the given resonance structure is shown in Figure 12.
(d)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given carbocation and its hybrid are,
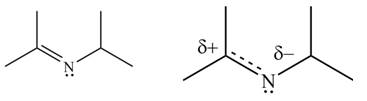
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
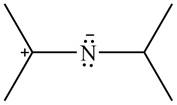
Figure 13
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
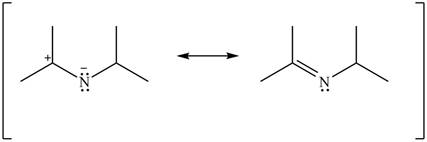
Figure 14
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
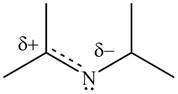
Figure 15
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
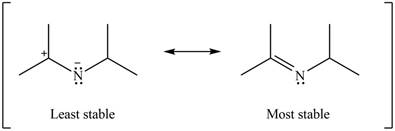
The given Figure 2 shows that the left side structure does not contain double bond. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.

Figure 16
The second resonance structure for given carbocation and its hybrid is shown in Figure 14 and 15. The increasing order of stability for the given resonance structure is shown in Figure 16.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Compound b is more polar than A. Explain. (Hint: draw resonance structures)arrow_forwarda. What is the hybridization of each N atom in nicotine? b. What is the geometry around each N atom? c. In what type of orbital does the lone pair on each N atom reside? d. Draw a constitutional isomer of nicotine. e. Draw a resonance structure of nicotine.arrow_forwardWhich of the given resonance structures (A, B, or C) contributes most tothe resonance hybrid? Which contributes least??arrow_forward
- This is an organic chem question. draw 2 additional resonance structures for each part a and barrow_forwardBreak compound A into several small (less or equals to 6 carbon atom) molecules. You need to explain the reasons why you choose to break those bonds.arrow_forwardLabel the resonance structures in each pair as major, minor, or equal contributors to the hybrid. Then draw the hybridarrow_forward
- Answer the following questions about erlotinib and terbinafine. Erlotinib,sold under the trade name Tarceva, was introduced in 2004 for thetreatment of lung cancer. Terbinafine is an antifungal medication used totreat ringworm and fungal nail infections. Draw two additional resonance structures for terbinafine that containall uncharged atomsarrow_forwardRizatriptan (trade name Maxalt) is a prescription drug used for the treatment of migraines. (a) How many aromatic rings does rizatriptan contain? (b) Determine the hybridization of each N atom. (c) In what type of orbital does the lone pair on each N reside? (d) Draw all the resonance structures for rizatriptan that contain only neutral atoms. (e) Draw all reasonable resonance structures for the five-membered ring that contains three N atoms.arrow_forwardSee the Attachment & solev the followings (a) Add curved arrows to show how the starting material A is convertedto the product B. (b) Draw all reasonable resonance structures for B. (c) Draw the resonance hybrid for B.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
