
Concept explainers
Write chemical equations, showing all necessary reagents, for the preparation of
by each of the following methods:
Hydroboration–oxidation of an
Use of a Grignard reagent
Use of a Grignard reagent in a way different from part (b)
Reduction of a
Hydrogenation of an
Reduction with sodium borohydride
Interpretation:
The chemical equations showing all necessary reagents for the preparation of
Concept introduction:
Hydroboration-oxidation of an alkene results in overall addition of water molecule across the double bond with a regioselectivity opposite to that of Markovnikov’s rule.
In the overall reaction, a hydrogen atom gets attached to the double bonded carbon atom having fewer hydrogens and the hydroxyl group gets attached to the carbon atom having greater number of hydrogens.
When a Grignard reagent reacts with formaldehyde primary alcohols are produced.
Grignard reagents are nucleophilic and react with oxiranes producing alcohols.
Aldehydes are reduced to the corresponding primary alcohols by using
By a suitable reducing agent like lithium aluminum hydride, carboxylic acids are reduced to the corresponding primary alcohols.
Answer to Problem 16P
Solution:
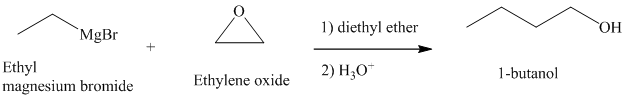

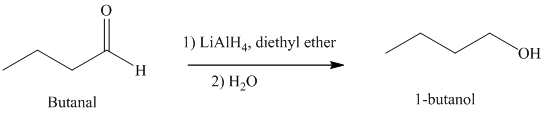
Explanation of Solution
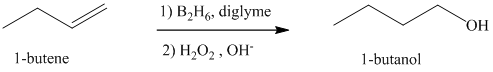
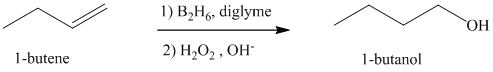
a) Hydroboration-oxidation of an alkene to form
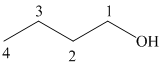
The structure of
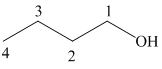
The hydroxyl group is attached to the C1 carbon atom. This alcohol is produced by hydroboration-oxidaton of an alkene. So in the alkene, the C1 carbon atom must be double bonded to the the C2 carbon atom. Thus, the alkene must be
The regioselectivity of hydroboration-oxidation is such that the hydrogen atom will get attached to the C2 carbon atom while the hydroxyl group will get attached to the C1 carbon atom producing
The reaction is shown below:
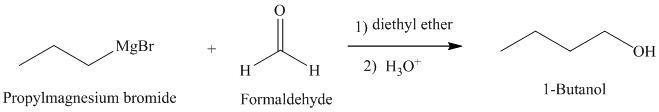
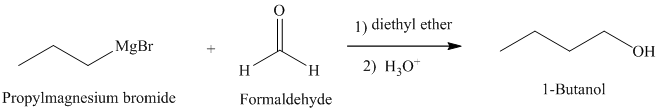
b) Grignard reagent is used to prepare
The structure of
Grignard reagents are nucleophilic and react with carbonyl groups forming a new carbon-carbon bond. An aqueous acid is used to convert the intermediate alkoxy ion to the corresponding alcohol.
In
The reaction is shown below:
c) Different Grignard reagent is used to prepare
The structure of
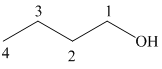
It is a primary alcohol. The hydroxyl group is attached to the C1 carbon atom.
Grignard reagents reacts with ethylene oxide to produce primary alcohol containing two more carbon atoms than the alkyl halide.
Thus, ethyl magnesium bromide, upon reaction with ethylene oxide, will produce
The reaction is shown below:
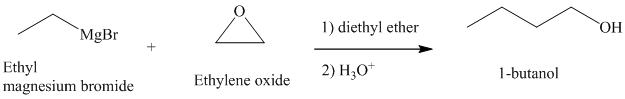
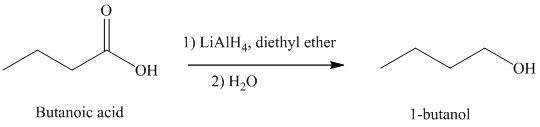
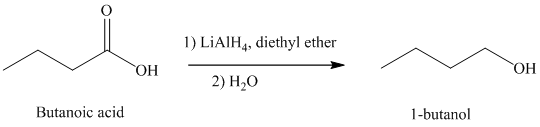
d) The reduction of carboxylic acid to produce

Carboxylic acids, upon reduction, produce the correspionding primary alcohols.
Thus, reduction of butanoic acid with Lithium aluminum hydride will produce
The reaction is shown below:
e) Hydrogenation of an aldehyde to form

Reduction of aldehydes with a suitable reducing agent produces the corresponding primary alcohols.
Thus, reduction of butanal in the presence of Lithium aluminum hydride will produce
The reaction is shown below:
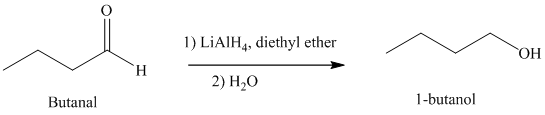
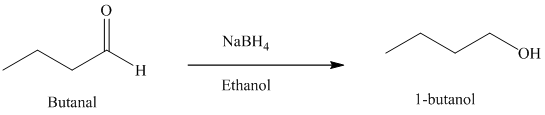
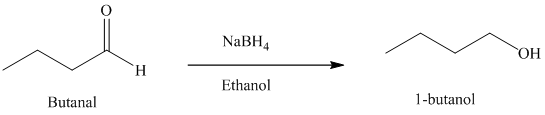
f) Reduction of an aldehyde to form

Sodium borohydride is used for the reduction of aldehydes and ketones to primary and secondary alcohols respectively.
Thus, reduction of butanal with sodium borohydride will produce
The reaction is shown below:
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Aldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forwardDraw the structural formula of the enol formed in the following alkyne hydration reaction and then draw the structural formula of the carbonyl compound with which this enol is in equilibrium.arrow_forwardWrite the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Dilute sulfuric acid (c) Diborane in diglyme, followed by basic hydrogen peroxide (d) Bromine in carbon tetrachloride (e) Bromine in water (f) Peroxyacetic acid (g) Ozone (h) Product of part (g) treated with zinc and water (i) Product of part (g) treated with dimethyl sulfide (CH3)2Sarrow_forward
- Starting with toluene (methylbenzene) and any other inorganic or organic reagents or catalysts, show a series of reactions (with reagents) for the synthesis of benzyl ethanoate (benzyl acetate).arrow_forwardDraw structural formulas for (1) the alkyltriphenylphosphonium salt formed by treatment of each haloalkane with triphenylphosphine, (2) the phosphonium ylide formed by treatment of each phosphonium salt with butyllithium, and (3) the alkene formed by treatment of each phosphonium ylide with acetone.arrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forward
- In the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. (a) Why are triphenylmethyl ethers so readily hydrolyzed by aqueous acid? (b) How might the structure of the triphenylmethyl group be modified to increase or decrease its acid sensitivity?arrow_forward(a) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Phenol (ii) Benzaldehyde and Acetophenone(b) An organic compound with molecular formula C5H10O does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Identify the compound and write all chemical equations for the reactions.arrow_forwardWhich of the following statements is false? One of the products of alkene epoxidation is carboxylic acid. Tertiary alcohols react with oxidizing agents such as chromate, to produce ketones. Primary alcohols react with oxidizing agents such as chromate, to produce aldehydes. Williamson Ether Synthesis involves the preparation of ethers using alkyl halides. None of the above.arrow_forward
- Draw the structural formula of the principal organic product formed when benzonitrile is treated with CH3MgBr followed by work-up with dilute acid.arrow_forwardWrite the chemical equation showing reactants, products and catalysts needed (if any) for thefollowing reactions. Write the IUPAC name of the product right beside the structure. a) Reaction of two phenol b) Reaction of 2-bromophenol with sodium hydroxide c) Reaction of potassium phenoxide with 2-chloropentane d) Reaction of isopropyl propyl ether with HBr e) Reaction of 3-methylpentan-3-ol with sulfuric acid f) Dehydration of 1,5-hepta-diolg) Oxidation of 1-decanolh) Hydration od hepta-2,3-dienearrow_forwardSynthesize 2-Methyl-4-heptanone from 2-methyl-1-propanol and butanal using the organic or inorganic reagents that are neededarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
