
Concept explainers
PRACTICE PROBLEM 16.1
(a) Give IUPAC substitutive names for the seven isomeric
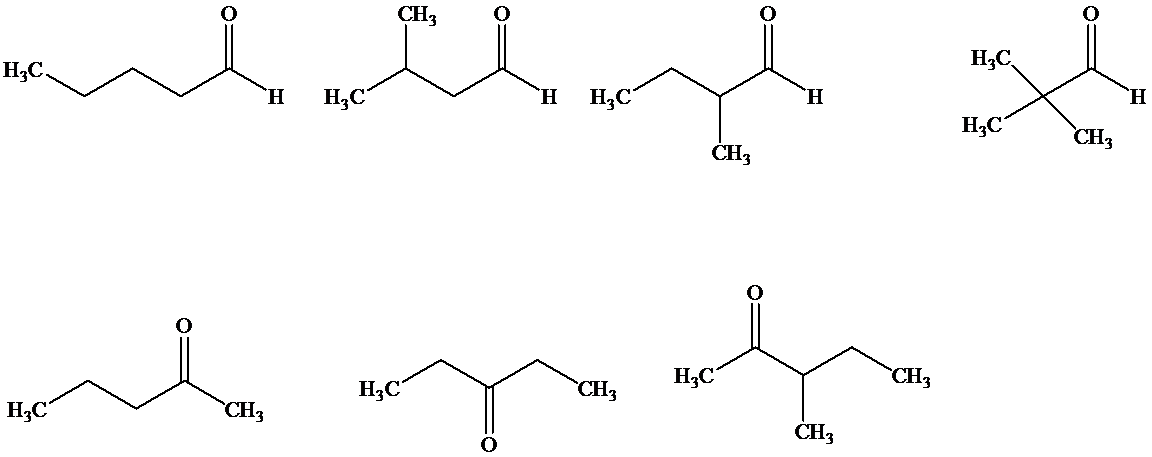
(b) Give structures and names (common or IUPAC substitutive names) for all the aldehydes and ketones that contain a benzene ring and have the formula
Interpretation:
For the given molecular formula,
Concept introduction:
Answer to Problem 1PP
Solution:
a)

b)
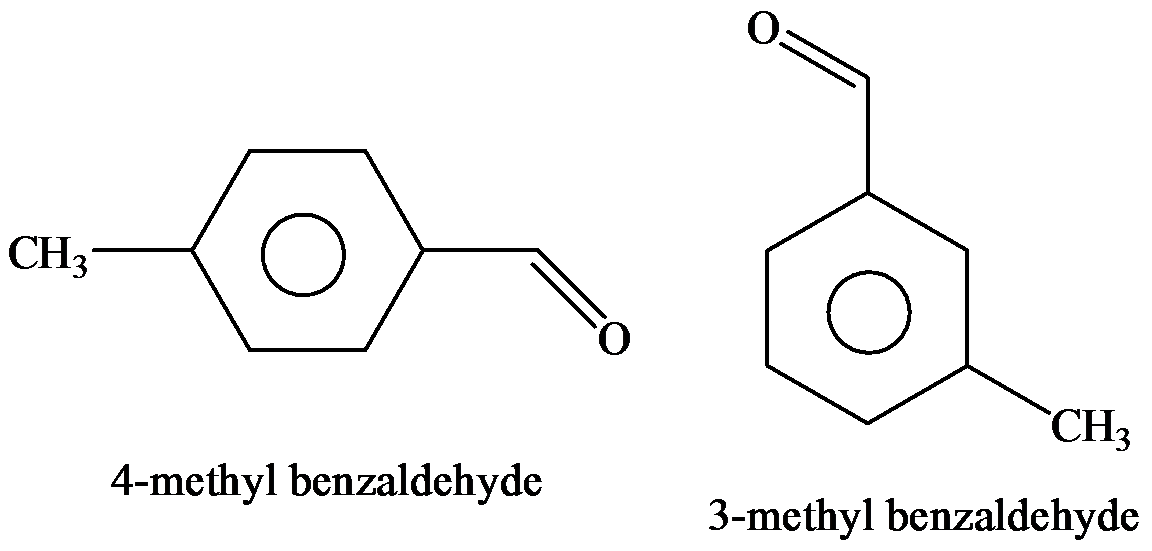
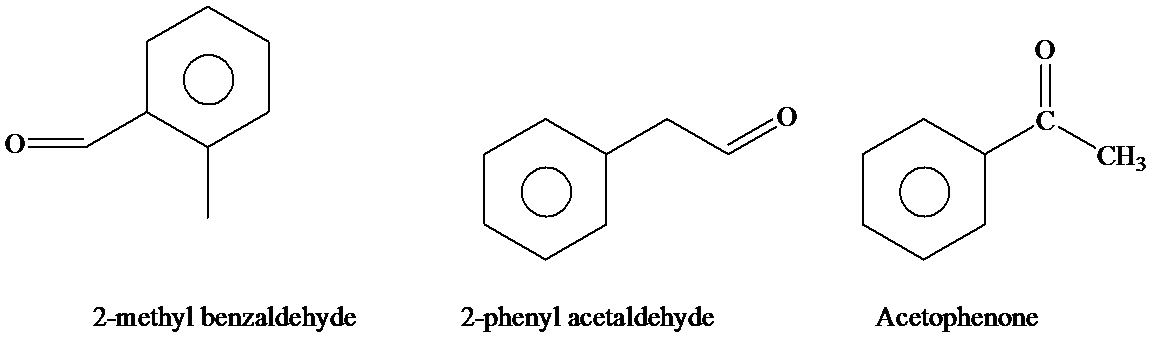
Explanation of Solution
Given information:
The molecular formula is
The IUPAC name is as follows:

Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry
Organic Chemistry (8th Edition)
Essential Organic Chemistry (3rd Edition)
Chemistry & Chemical Reactivity
General, Organic, and Biological Chemistry (3rd Edition)
Organic Chemistry As a Second Language: Second Semester Topics
- Wolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forwardStarting exactly with any acid chloride with exactly with 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules: (a) 2,6-dimethyl-4-heptanone (b) 4-propyl-4-octanolarrow_forward(a) Illustrate the following name reactions by giving example :(i) Cannizzaro’s reaction(ii) Clemmensen reduction(b) An organic compound A contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the possible structure of compound A.arrow_forward
- (Give clear handwritten solution) Which compound you predict as a major product when reacting benzenediazonium chloride with phenol?...arrow_forwardStarting with acid chloride with exactly 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules:arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)m-Nitrobenzenesulfonic acidarrow_forward
- Compound F may be synthesised by the method attached: When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forwardgive the structural formula for compounds A to Garrow_forward(−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from Atropa belladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated. (a) Explain this result by drawing a stepwise mechanism. (b) Explain why littorine, an isomerisolated from the tailower plant in Australia, can be obtained optically pure regardless of the amount of base used during isolation.arrow_forward
- A certain compound is known to contain an aromatic benzene ring but failed to produce a fragrant yellow solution upon subjecting it to the nitration test. What may be a possible explanation for this? A. The benzene ring is part of a highly conjugated, blue dye molecule. B. The benzene ring contains a strong electron-withdrawing group. C. The benzene ring has no available sites left for electrophilic attack. D. All of the given. Kindly explain your answer in detail.arrow_forwardMyristoleic acid, C14H26O2, yields myristic acid on catalytic hydrogenation and undergoes oxidative cleavage with ozone to yield pentanoic acid and nonanedioic acid. Draw the structure of Myristoleic acid.arrow_forwardCompound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over a palladium catalyst to give B(C9H18). On ozonolysis, compound A gave, among other things, a ketone that was identified as cyclohexanone. Ontreatment with NaNH2 in NH3, followed by addition of iodomethane, compound A gave a new hydrocarbon, C(C10H14 ) . What are the structures of A, B, a nd C?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
