
Concept explainers
Show how each of the following compounds can be synthesized from cyclopentanol and any necessary organic or inorganic reagents. In many cases the desired compound can be made from one prepared in an earlier part of the problem.
trans-
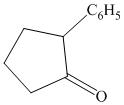
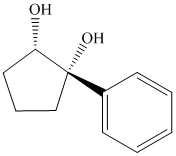
Interpretation:
Each of the given product is to be synthesized from cyclopentanol and necessary organic or inorganic reagents.
Concept introduction:
Alcohols can be prepared from a variety of reagents.
Reaction of Grignard reagents with carbonyl compounds produces the corresponding alcohols.
Grignard reagents also react with oxiranes to produce alcohols.
The allylic and benzylic carbon atoms are selectively brominated using NBS reagent.
Alcohols undergo dehydration in acidic medium, producing alkenes. These alkenes can be converted to diols using osmium tetra oxide.
Answer to Problem 23P
Solution:
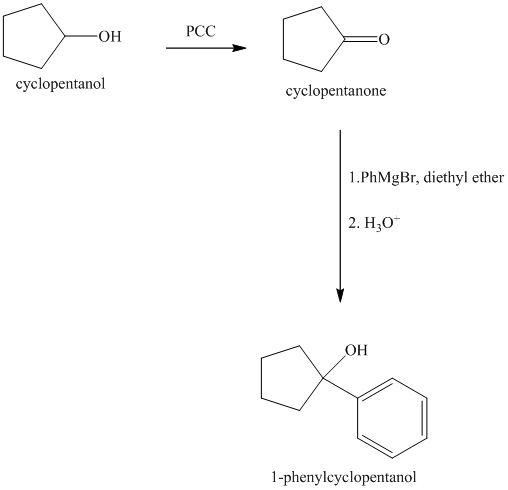
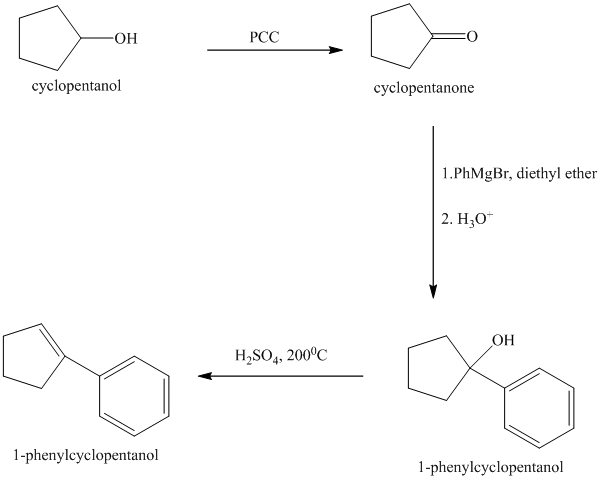
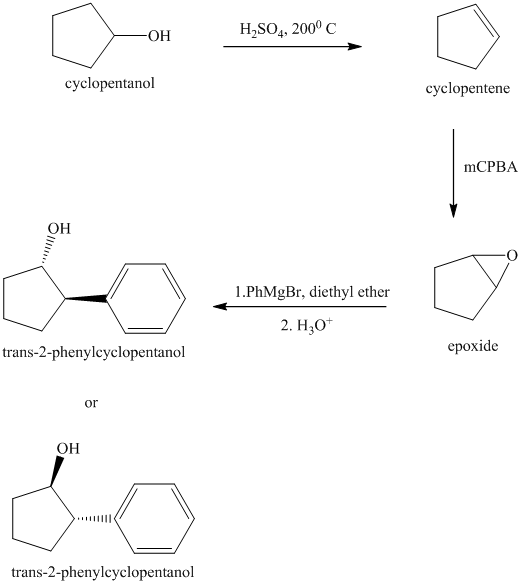
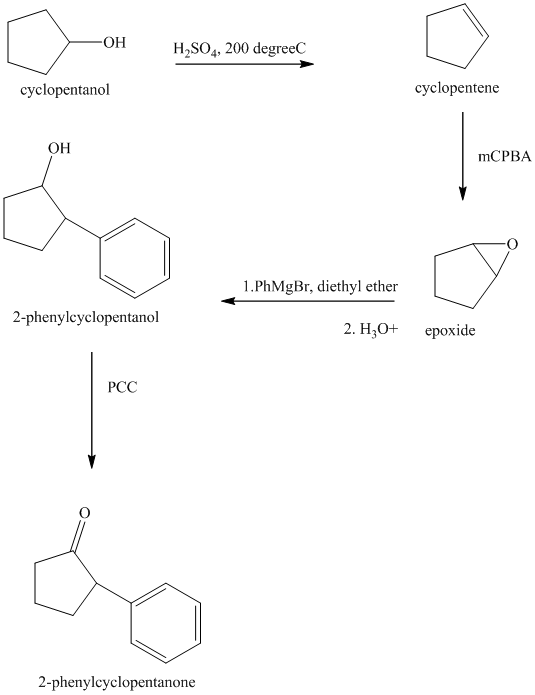
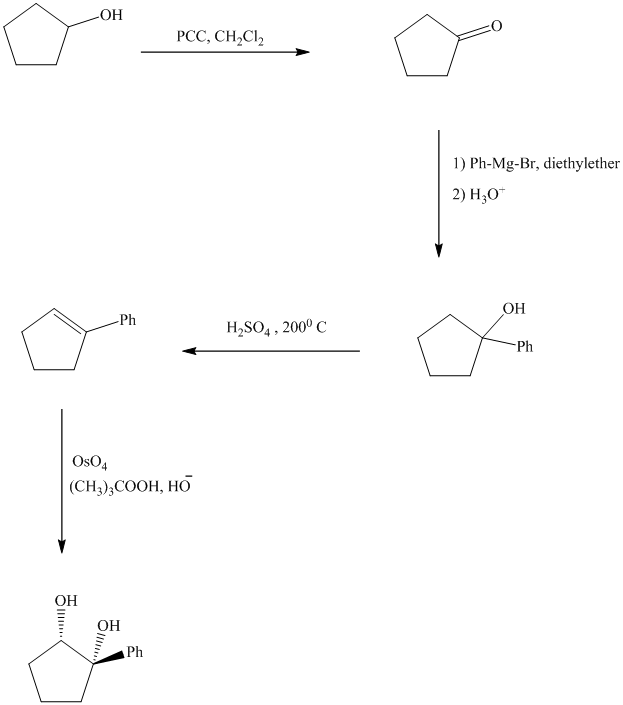
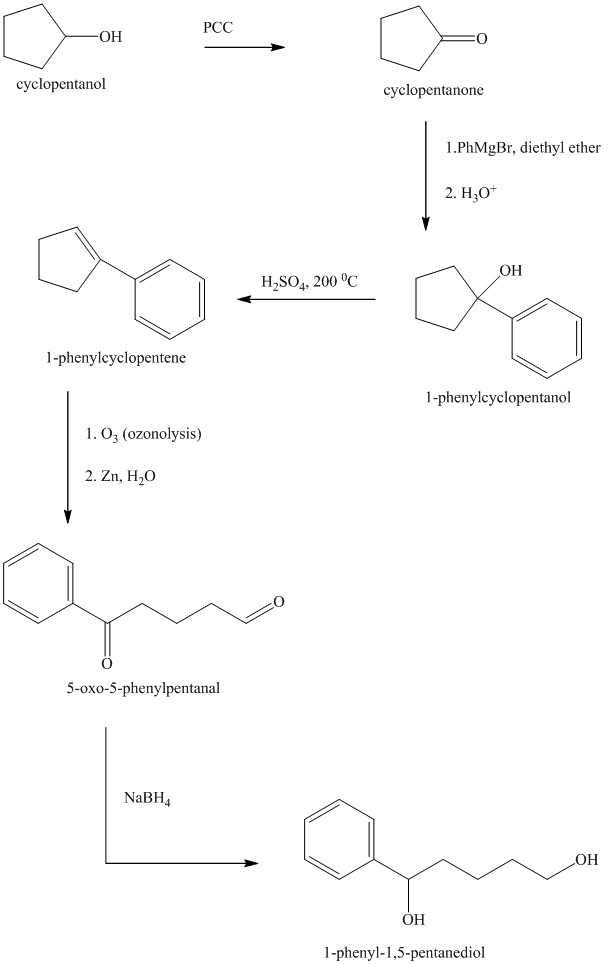
Explanation of Solution
Synthesis of
The structure for cyclopentanol and
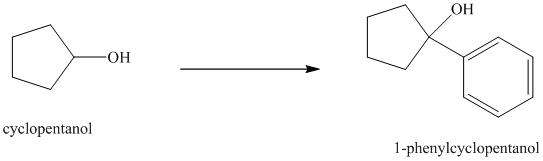
In

Synthesis of
The structure for cyclopentanol and
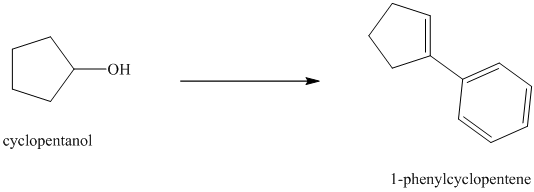
In
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
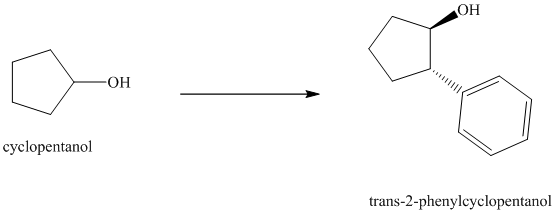
In
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
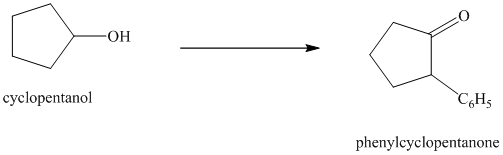
In
The sequence of reactions starting from cyclopentanol to yield the final given product
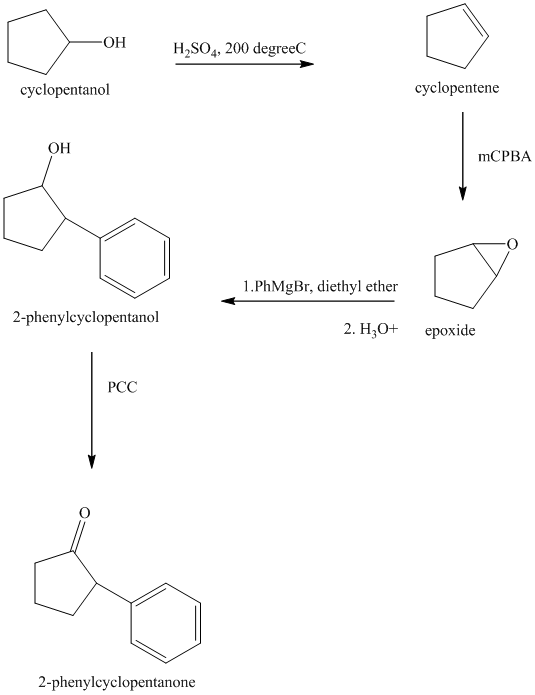
Synthesis of the given diol from cyclopentanol.
The structure for the given diol is as follows:
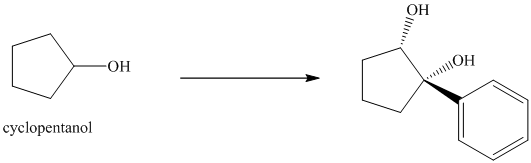
In the given diol, one phenyl ring and one hydroxyl group are attached to the same carbon of cyclopentane ring. The other hydroxyl group is attached to C2 position of cyclopentane ring.
Oxidation of the cyclopentanol will produce cyclopentanone. Reaction of this cyclopentanone with phenyl magnesium bromide will form a tertiary alcohol. Acid catalyzed dehydration of this tertiary alcohol will produce
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
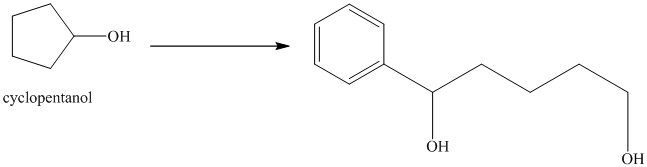
Cyclopentanol, when undergoes oxidation in presence of a mild oxidizing agent such as pyridinium chlorochromate in dichlormethane, the hydroxyl group turns to a carbonyl group and forms cyclopentanone. Cyclopentanone, when treated with Grignard reagent (PhMgBr) in the presence of diethyl ether with acidic workup, forms
The sequence of reactions is shown below.

Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry - Standalone book
- Starting with cyclohexanone, show how to prepare these compounds. In addition to the given starting material, use any other organic or inorganic reagents as necessary. a. Cyclohexanol b. Cyclohexene c. cis-1,2-Cyclohexanediol d. 1-Methylcyclohexanol e. 1-Methylcyclohexene f. 1-Phenylcyclohexanol g. 1-Phenylcyclohexene h. Cyclohexene oxide i. trans-1,2-Cyclohexanediolarrow_forwardUsing cyclohexanone as the starting material, describe how each of the following compounds can be synthesized:arrow_forwardReaction of 2-methyl-1-hexene with 1) m-Chloroperbenzoic acid followed by 2) addition of hydronium ion will provide Group of answer choices a) an enantiomeric mixture of 2-methyl-1,2-hexanediol b) an enantiomeric mixture of 2-methyl-2,3-hexanediol c) 2-methylhexan-2-ol d) 2-methyl-1,2-hexanediol e) an enantiomeric mixture of 2-methylhexan-2-olarrow_forward
- Show how the following transformations may be accomplished in good yield. You may use any additional reagents that are needed.(a) benzoic acid - phenyl cyclopentyl ketone (b) 1-bromohept-2-ene - oct-3-enalarrow_forwardShow how each of the following compounds can be synthesized from benzene: a. o-nitrophenol b. p-nitroaniline c. p-bromoanisole d. anisolearrow_forwardIndicate how the following compound can be synthesized from the given starting material and any other necessary reagent:arrow_forward
- Aside from the specified main starting material, only the following compounds can be used as sources of the carbon backbone for the target compound: acetaldehyde ethanol propanoic acid acetic acid ethyl bromoacetate propan-1-ol benzene (2E,4E)-hexa-2,4-diene propan-2-ol buta-1,3-diene methanol propan-2-one (acetone) (chloromethyl)benzene (benzyl chloride) 5-methyloxolan-2-one sodium cyanide 1-(chloromethyl)-4-methylbenzene phenylacetaldehyde sodium methoxide diethyl propanedioate (diethyl malonate) piperidine sodium ethoxide - There are no restrictions with regards to the use of other reagents such as catalysts, solvents, redox agents, protection/deprotection agents, etc. as long as they will not be used as sources of carbon for the skeleton of the target compound. 1. Design a retrosynthetic map and from it, propose a multi-step synthetic approach for the preparation of the target compound from the specified starting material.…arrow_forwardAside from the specified main starting material, only the following compounds can be used as sources of the carbon backbone for the target compound: acetaldehyde ethanol propanoic acid acetic acid ethyl bromoacetate propan-1-ol benzene (2E,4E)-hexa-2,4-diene propan-2-ol buta-1,3-diene methanol propan-2-one (acetone) (chloromethyl)benzene (benzyl chloride) 5-methyloxolan-2-one sodium cyanide 1-(chloromethyl)-4-methylbenzene phenylacetaldehyde sodium methoxide diethyl propanedioate (diethyl malonate) piperidine sodium ethoxide There are no restrictions with regards to the use of other reagents such as catalysts, solvents, redox agents, protection/deprotection agents, etc. as long as they will not be used as sources of carbon for the skeleton of the target compound. Design a retrosynthetic map and from it, propose a multi-step synthetic approach for the preparation of the target compound from the specified starting material.…arrow_forwardAside from the specified main starting material, only the following compounds can be used as sources of the carbon backbone for the target compound: acetaldehyde ethanol propanoic acid acetic acid ethyl bromoacetate propan-1-ol benzene (2E,4E)-hexa-2,4-diene propan-2-ol buta-1,3-diene methanol propan-2-one (acetone) (chloromethyl)benzene (benzyl chloride) 5-methyloxolan-2-one sodium cyanide 1-(chloromethyl)-4-methylbenzene phenylacetaldehyde sodium methoxide diethyl propanedioate (diethyl malonate) piperidine sodium ethoxide There are no restrictions with regards to the use of other reagents such as catalysts, solvents, redox agents, protection/deprotection agents, etc. as long as they will not be used as sources of carbon for the skeleton of the target compound. Design a retrosynthetic map and from it, propose a multi-step synthetic approach for the preparation of the target compound from the specified starting material. USE…arrow_forward
- How will you synthesize cyclohexanecarboxaldehyde (cyclohexylmethanal) from the following reagents? (There are no restrictions on the reagents or the number of steps). (a) Cyclohexanone (b) Ethynylcyclohexane (c) Methyl cyclohexylformate (Remember: Formic acid is the IUPAC recognized name for Methanoic acid) (d) Cyclohexanecarboxylic acid (Cyclohexylmethanoic acid) (e) Vinylcyclohexanearrow_forwardChoose a Grignard reagent _______and a ketone _______ that can be used to produce the following compound: 1-Ethylcyclohexanolarrow_forwardShow how the following compounds will be synthesized, beginning with benzene or toluene and any reagents required. Assume para, in ortho, para mixtures, are the main ingredients (and separable from ortho). 1) p-bromobenzene sulfonic acid 2) 1-phenypentane 3) m-chloronitrobenzene 4) o-chlorobenzoic acidarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

