
Concept explainers
Draw the structure for each of the following:
- a. isobutyraldehyde
- b. 4-hexenaI
- c. diisopentyl
ketone - d. 3-methylcyclohexanone
- e. 2.4-pentanedione
- f. 4-bromo-3-heptanone
- g. γ-bromocaproaldehyde
- h. 2-ethylcyclopentanecarbaldehyde
- i. 4-methyl-5-oxohexanal
(a)
Interpretation:
The structure of Isobutyraldehyde has to be drawn.
Answer to Problem 53P
The structure of Isobutyraldehyde is given below:
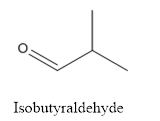
Explanation of Solution
The compound has aldehyde group that is attached to the isobutyl group.
The structure of isobutyraldehyde is as follows.

(b)
Interpretation:
The structure of 4-hexenal has to be drawn.
Answer to Problem 53P
The structure of 4-hexenal is given below:
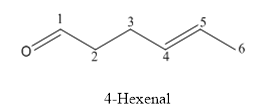
Explanation of Solution
The compound has aldehyde functional group and double bond at the 4th position of the compound.
The structure is as follows.

(c)
Interpretation:
The structure of diisopentyl ketone has to be drawn.
Answer to Problem 53P
The structure of diisopentyl ketone is given below:
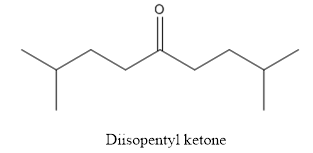
Explanation of Solution
The compound has ketone functional group. Two isopentyl groups are attached to the keto group.
The structure of diisopentyl ketone is as follows.

(d)
Interpretation:
The structure of 3-methylcyclohexanone has to be drawn.
Answer to Problem 53P
The structure of 3-methylcyclohexanoneis given below:
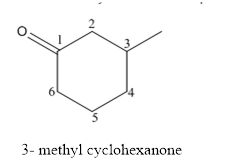
Explanation of Solution
The compound has ketone functional and group and it is called as cyclohexanone and it has one methyl group at 3rd position of the ring.
The structure of 3-methyl cyclohexanone is as follows.

(e)
Interpretation:
The structure of 2,4-pentanedione has to be drawn.
Answer to Problem 53P
The structure of 2,4-pentanedione is given below:
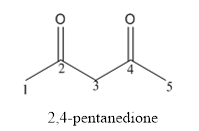
Explanation of Solution
The compound has two keto functional groups and these are attached to the pentane carbon chain.

(f)
Interpretation:
The structure of 4-bromo-3-heptanone has to be drawn.
Answer to Problem 53P
The structure of 4-bromo-3-heptanone is given below:
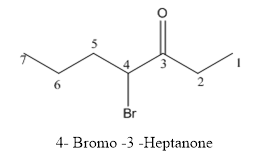
Explanation of Solution
The compound has keto functional group and it is attached to the heptanes carbon chain. At the 4th position of the carbon chain has bromine attachment.

(g)
Interpretation:
The structure of
Answer to Problem 53P
The structure of
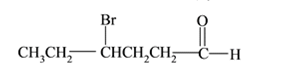
Explanation of Solution
Compound has aldehyde functional group and at the
The structure of
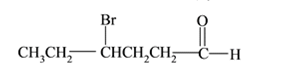
(h)
Interpretation:
The structure of 2-ethylcyclopentanecarbaldehyde has to be drawn.
Answer to Problem 53P
The structure of 2-ethylcyclopentanecarbaldehyde is given below:
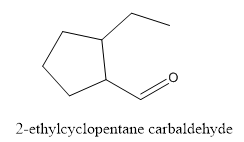
Explanation of Solution
In this compound, pentane ring has ethyl group and aldehyde functional group.
The structure of 2-ethylcyclopentane carbaldehyde.

(i)
Interpretation:
The structure of 4-methyl-5-oxohexanal has to be drawn.
Answer to Problem 53P
The structure of 4-methyl-5-oxohexanal is givenbelow:
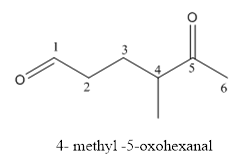
Explanation of Solution
The compound has aldehyde functional group at the 5th position of the carbon chain as keto group it is named as oxo-because it is referred to an attachment.
The structure of 4- methyl-5-oxohexanal.

Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY-PACKAGE
- Based on the following samples, which is most readily oxidized? a. n-butyl alcohol b. sec-butyl alcohol c. tert-butyl alcohol d. Acetaldehyde e. Benzaldehyde f. Acetone g. Acetophenone h. Isopropyl alcoholarrow_forwardIzopropanole doesn't form by Select one: a. Hydration of propene b. Reduction of propanal c. Hydration of 2-chloropropane d. Reduction of propan-2-onearrow_forward1. Write the skeletal structures of propanal, acetone and cyclohexanone. What is the major intermolecular force (IMF) found in them? Based on their major intermolecular force and molecular weight, what can you predict on their solubility in water? Chemical Name Skeletal Structures Major IMF Solubility in water Propanal Acetone Cyclohexanone 2. What is the purpose of Tollens’ test (Part B)? What is the evidence of a positive result? 3. What is the purpose of oxidation test (Part C)? What is the evidence of a positive result?arrow_forward
- N-Chlorosuccinimide (NCS) serves as a source of Cl+ in electrophilic addition reactions to alkenes and alkynes. Keeping this in mind, draw a stepwise mechanism for the following addition to but-2-yne.arrow_forward39. Identify the type of the double bond given in each structure: E or Z type.arrow_forwardReview the oxidation reactions using Cr6+reagents. Then draw the product formed when compound B is treated with following reagent. PCCarrow_forward
- Matching Type 1. A tertiary alkyl halide 2. Most soluble ROH in water 3. Has vinegar like odor 4. Blue in llitmus paper test 5. Most commonly used organic solvent A. 2-methyl-2-fluoropropane B. Isobutanol C. Diethyl ether D. Ethanamide E. Chlorobenzene F. Ethanoic acid G. Hexyl propanoatearrow_forwardOxidation of 1-butanol and 2-butanol yields (respectively) a. butanal exclusively b. 2-butanone exclusively c. butanal and 2-butanone d. butanal and 2-butanone if PCC is the oxidizing agent e. butanal and 2-butanone if the Jones reagent is the oxidizing agentarrow_forwardDraw the structure for each of the following: a. isobutyraldehyde d. 3-methylcyclohexanone g. g-bromocaproaldehyde b. 4-hexenal e. 2,4-pentanedione h. 2-ethylcyclopentanecarbaldehyde c. diisopentyl ketone f. 4-bromo-3-heptanone i. 4-methyl-5-oxohexanalarrow_forward
- QVI: Starting from benzene as the only organic reagent you have and use any other inorganic reagents to preparearrow_forwardConvert benzene into attached compound. You may also use any inorganicreagents and organic alcohols having four or fewer carbons. One step of the synthesis must use a Grignard reagent.arrow_forwardmajor product of dehydration reaction using H2SO4arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



