
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 88AP
. The following are representations of acid-base reactions:
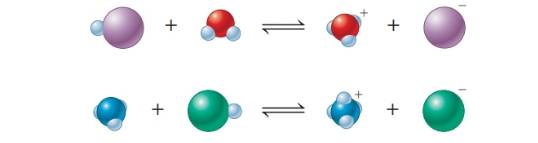
a. Label each of the species in both equations as an acid or base, and explain.
b. For those species that are acids, which labels apply:
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 16 Solutions
Introductory Chemistry: A Foundation
Ch. 16.1 - Exercise 16.1 Which of the following represent...Ch. 16.2 - Vinegar contains acetic acid and is used in salad...Ch. 16.3 - Exercise 16.2 Calculate [H+] in a solution in...Ch. 16.4 - Prob. 16.3SCCh. 16.4 - Prob. 1CTCh. 16.4 - Prob. 16.4SCCh. 16.4 - Exercise 16.5 The pH of rainwater in a polluted...Ch. 16.4 - Exercise 16.6 The pOH of a liquid drain cleaner...Ch. 16.5 - Exercise 16.7 Calculate the pH of a solution of...Ch. 16 - You are asked for the H+ concentration in a...
Ch. 16 - Explain why Cl- does not affect the pH of an...Ch. 16 - Write the general reaction for an acid acting in...Ch. 16 - Differentiate among the terms concentrated,...Ch. 16 - What is meant by “pH”? True or false: A strong...Ch. 16 - Consider two separate solutions: one containing a...Ch. 16 - Prob. 7ALQCh. 16 - Prob. 8ALQCh. 16 - Stanley’s grade-point average (GPA) is 3.28. What...Ch. 16 - Prob. 10ALQCh. 16 - . Mixing together aqueous solutions of acetic acid...Ch. 16 - Prob. 12ALQCh. 16 - . Consider the equation:...Ch. 16 - . Choose the answer that best completes the...Ch. 16 - Prob. 15ALQCh. 16 - . The following figures are molecular-level...Ch. 16 - Prob. 17ALQCh. 16 - What are some physical properties that...Ch. 16 - Write an equation showing how HCl(g) behaves as an...Ch. 16 - Prob. 3QAPCh. 16 - How do the components of a conjugate acid—base...Ch. 16 - 5. Given the general equation illustrating the...Ch. 16 - According to Arrhenius, ____________ produce...Ch. 16 - Which of the following do not represent a...Ch. 16 - Which of the following do not represent a...Ch. 16 - In each of the following chemical equations,...Ch. 16 - . In each of the following chemical equations,...Ch. 16 - . Write the conjugate acid for each of the...Ch. 16 - . Write the conjugate acid for each of the...Ch. 16 - Prob. 13QAPCh. 16 - . Write the conjugate base for each of the...Ch. 16 - . Write a chemical equation showing how each of...Ch. 16 - . Write a chemical equation showing how each of...Ch. 16 - . What does it mean to say that an acid is strong...Ch. 16 - Prob. 18QAPCh. 16 - . How is the strength of an acid related to the...Ch. 16 - . A strong acid has a weak conjugate base, whereas...Ch. 16 - . Write the formula for the hydronium ion. Write...Ch. 16 - Prob. 22QAPCh. 16 - . Organic acids contain the carboxyl group Using...Ch. 16 - Prob. 24QAPCh. 16 - 25. Which of the following acids have relatively...Ch. 16 - . The “Chemistry in Focus” segment Plants Fight...Ch. 16 - . Water is the most common amphoteric substance,...Ch. 16 - . Anions containing hydrogen (for example. HCO3and...Ch. 16 - . What is meant by the iou-product constant for...Ch. 16 - . What happens to the hydroxide ion concentration...Ch. 16 - Prob. 31QAPCh. 16 - Prob. 32QAPCh. 16 - . Calculate the [OH-] in each of the following...Ch. 16 - . Calculate the [OH-] in each of the following...Ch. 16 - 35. For each pair of concentrations, tell which...Ch. 16 - . For each pair of concentrations, tell which...Ch. 16 - . Why do scientists tend to express the acidity of...Ch. 16 - . Using Fig. 16.3, list the approximate pH value...Ch. 16 - . For a hydrogen ion concentration of 2.33106M,...Ch. 16 - . The “Chemistry in Focus” segment Garden-Variety...Ch. 16 - . Calculate the pH corresponding to each of the...Ch. 16 - Prob. 42QAPCh. 16 - Prob. 43QAPCh. 16 - Prob. 44QAPCh. 16 - Prob. 45QAPCh. 16 - . Calculate the pOH value corresponding to each of...Ch. 16 - . For each hydrogen ion concentration listed,...Ch. 16 - . For each hydrogen ion concentration listed,...Ch. 16 - . Calculate the hydrogen ion concentration, in...Ch. 16 - . Calculate the hydrogen ion concentration, in...Ch. 16 - . Calculate the hydrogen ion concentration, in...Ch. 16 - . Calculate the hydrogen ion concentration, in...Ch. 16 - . Calculate the pH of each of the following...Ch. 16 - Prob. 54QAPCh. 16 - 55. When 1 mole of gaseous hydrogen chloride is...Ch. 16 - . A bottle of acid solution is labeled “3 M HNO3.”...Ch. 16 - . Calculate the hydrogen ion concentration and the...Ch. 16 - . Calculate the pH of each of the following...Ch. 16 - . What characteristic properties do buffered...Ch. 16 - Prob. 60QAPCh. 16 - . Which component of a buffered solution is...Ch. 16 - Prob. 62QAPCh. 16 - . Which of the following combinations would act as...Ch. 16 - . A buffered solution is prepared containing...Ch. 16 - . The concepts of acid-base equilibria were...Ch. 16 - . Strong buses are bases that completely ionize in...Ch. 16 - Prob. 67APCh. 16 - Prob. 68APCh. 16 - Prob. 69APCh. 16 - Prob. 70APCh. 16 - Prob. 71APCh. 16 - Prob. 72APCh. 16 - Prob. 73APCh. 16 - Prob. 74APCh. 16 - 75. A conjugate acid-base pair Consists of two...Ch. 16 - . Acetate ion, C2H3O2- , has a stronger affinity...Ch. 16 - Prob. 77APCh. 16 - Prob. 78APCh. 16 - Prob. 79APCh. 16 - Prob. 80APCh. 16 - Prob. 81APCh. 16 - Prob. 82APCh. 16 - Prob. 83APCh. 16 - Prob. 84APCh. 16 - . A(n) _________ solution contains a conjugate...Ch. 16 - . When sodium hydroxide, NaOH, is added dropwise...Ch. 16 - . When hydrochloric acid, HCI. is added dropwise...Ch. 16 - . The following are representations of acid-base...Ch. 16 - . In each of the following chemical equations,...Ch. 16 - Prob. 90APCh. 16 - . Write the conjugate base for each of the...Ch. 16 - . Of the following combinations, which would act...Ch. 16 - Prob. 93APCh. 16 - . Calculate [H+] in each of the following...Ch. 16 - Prob. 95APCh. 16 - . Calculate the pH corresponding to each of the...Ch. 16 - Prob. 97APCh. 16 - Prob. 98APCh. 16 - Prob. 99APCh. 16 - . For each hydrogen or hydroxide ion concentration...Ch. 16 - . Calculate the hydrogen ion concentration, in...Ch. 16 - Prob. 102APCh. 16 - Prob. 103APCh. 16 - Prob. 104APCh. 16 - . Write the formulas for three combinations of...Ch. 16 - . Choose pairs in which the species listed first...Ch. 16 - . Complete the table for each of the following...Ch. 16 - . Consider 0.25 M solutions of the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the autoionization of liquid ammonia: Label each of the species in the equation as an acid or a base and explain your answer.arrow_forwardWrite equations to illustrate the acid-base reaction when each of the following pairs of Brnsted acids and bases are combined: Acid Base a.HOCl H2O b.HClO4 NH3 c.H2O NH2 d.H2O OCl e.HC2O4 H2Oarrow_forwardWrite a brief description of the relationships among each of the following groups of terms or phrases. Answers to the Concept-Linking Exercises are given at the end of the chapter. Terms from optional sections 17.1 and 17.3 are in italics. Exercise 11 covers terms related to logarithms, and it is also optional. Arrhenius acid, Brnsted-Lowry acid, Lewis acidarrow_forward
- a Write a net ionic equation to show that hypochlorous acid behaves as a Brnsted-Lowry acid in water. b The substance triethanolamine is a weak nitrogen-containing base, like ammonia. Write a net ionic equation to show that triethanolamine, C6H15O3N, behaves as a Brnsted-Lowry base in water.arrow_forwardThe following are representations of acidbase reactions: a. Label each of the species in both equations as an acid or a base and explain your answers. b. For those species that are acids, which labels apply: Arrhenius acid, BrnstedLowry acid, Lewis acid? What about the bases?arrow_forwardFor oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY