
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16.3, Problem 16.7P
Arrange the following compounds in order of increasing boiling point. Explain why you placed them in that order.
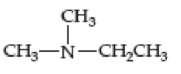
- (a) CH3CH2CH2CH2OH
- (b) CH3CH2CH2CH2NH2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Arrange the following compounds in the increasing order of their boiling points :CH3CHO, CH3CH2OH, CH3OCH3, CH3COOH.
Draw all possible carboxylic acids with the formula C5H10O2.
The following equation shows the reaction of baking soda (NaHCO3) and hydrochloric acid (HCl).
NaHCO3+HCl → CO2+H2O+NaCl
If you have 3.0 grams of NaHCO3, how many moles of HCl are needed for a complete relation?
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 16.2 - Identify the following compounds as primary,...Ch. 16.2 - Prob. 16.2PCh. 16.2 - Prob. 16.3PCh. 16.2 - Prob. 16.4PCh. 16.2 - Prob. 16.5KCPCh. 16.2 - Prob. 16.6KCPCh. 16.3 - Arrange the following compounds in order of...Ch. 16.3 - Draw the structures of (a) ethylamine and (b)...Ch. 16.4 - Provide compounds that fit the following...Ch. 16.4 - Prob. 16.10P
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Write an equation for the acid-base equilibrium...Ch. 16.5 - Prob. 16.13PCh. 16.5 - Prob. 16.14PCh. 16.5 - Prob. 16.15PCh. 16.5 - Prob. 16.16PCh. 16.6 - Prob. 16.17PCh. 16.6 - Prob. 16.18PCh. 16.6 - Prob. 16.19PCh. 16.6 - Prob. 16.20PCh. 16.6 - Prob. 16.21PCh. 16.6 - Prob. 16.22PCh. 16.7 - Prob. 16.1CIAPCh. 16.7 - Prob. 16.2CIAPCh. 16.7 - Prob. 16.3CIAPCh. 16 - (a) For the compound above, identify each nitrogen...Ch. 16 - The structure of the amino acid lysine (in its...Ch. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - Prob. 16.27UKCCh. 16 - Complete the following equations: (a) (b)...Ch. 16 - Prob. 16.29APCh. 16 - Draw the structures corresponding to the following...Ch. 16 - Name the following amines, and classify them as...Ch. 16 - Name the following amines, and identify them as...Ch. 16 - Prob. 16.33APCh. 16 - Which is a stronger base, diethyl ether or...Ch. 16 - Prob. 16.35APCh. 16 - Prob. 16.36APCh. 16 - The compound lidocaine is used medically as a...Ch. 16 - Prob. 16.38APCh. 16 - Draw the structures of the ammonium ions formed...Ch. 16 - Prob. 16.40APCh. 16 - Prob. 16.41APCh. 16 - Prob. 16.42APCh. 16 - Prob. 16.43APCh. 16 - Prob. 16.44APCh. 16 - Prob. 16.45CPCh. 16 - Prob. 16.46CPCh. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48CPCh. 16 - How do amines differ from analogous alcohols in...Ch. 16 - Name at least two undesirable characteristics are...Ch. 16 - Prob. 16.52CPCh. 16 - Complete the following equations (Hint: Answers...Ch. 16 - Prob. 16.54CPCh. 16 - Prob. 16.55CPCh. 16 - Why is cyclohexylamine not considered to be a...Ch. 16 - Prob. 16.57CPCh. 16 - Prob. 16.58GPCh. 16 - 1-Propylamine, 1-propanol, acetic acid, and butane...Ch. 16 - Prob. 16.60GPCh. 16 - Lemon juice, which contains citric acid, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the empirical formula for C3H6O3? C3H6O3 C6H12O6 CH2O None of thesearrow_forwardThe liquids butan-1-ol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.arrow_forwardConsider the following acids and their ionization constant, determine which conjugate base is HCOOH Ka = 1.7 x 10-4 (b) HCN Ka = 4.9 x 10-10arrow_forward
- Identify the acid on the left and its conjugate base on the right in the following equations:(a) HOCl + H2O ↔ H3O+ + OCl-(b) HONH2 + H2O ↔ HONH3+ + OH-(c) NH4+ + H2O ↔ NH3 + H3O+(d) 2HCO3-2 ↔ H2CO3 + CO3-2 (e) PO4-3 + H2PO4- ↔ 2HPO4-2arrow_forwardIf an unknown solution of cobalt (II) chloride has an absorbance of 0.79, what is its concentration? Include proper units, please How did you determine this using the Beer’s Law plot?arrow_forwardWhat mass of gallium oxide, Ga2O3, can be prepared from 29.0 g of gallium metal? The equation for the reaction is 4Ga + 3O2 ⟶ 2Ga2 O3.arrow_forward
- The standard heat of combustion of liquid methyl cyclopentane, C6H12(l),C6H12(l), was measured to be −3937.7 kJ/mol.−3937.7 kJ/mol. What is Δ?̂ ∘f C6H12(l),ΔH^f C6H12(l)∘, the standard heat of formation of liquid methyl cyclopentane?arrow_forwardWhat mass of nitrogen monoxide would be produced by complete reaction of 17.0 g of ammonia?arrow_forwardFor the following reactions, identify the atom(s) being oxidized and reduced:arrow_forward
- The chemical formula for deoxyribose is C___ H___O____.arrow_forwardWhat coefficients must be placed in the following blanks sothat all atoms are accounted for in the products?C6H12O6 S _________ C2H6O + _________ CO2(A) 2; 1(B) 3; 1(C) 1; 3(D) 2; 2arrow_forwardPropanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 486 K, whereas that of methyl acetate is 330 K. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license