
(a)
Interpretation:
The structure of the given compound has to be determined.
Concept introduction:
In chemistry Structure is the arrangement of
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
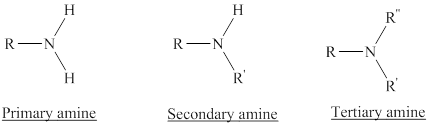
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(b)
Interpretation:
The structure of the given compound has to be determined.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
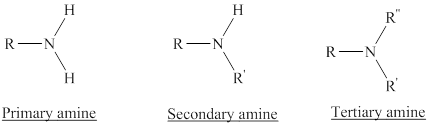
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(c)
Interpretation:
The structure of the given compound has to be determined.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
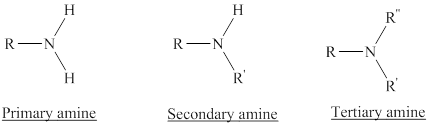
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(d)
Interpretation:
The structure of the given compound has to be determined.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
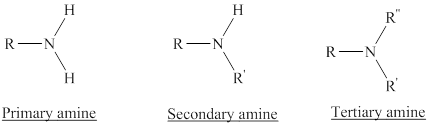
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Consider the following acids and their ionization constant, determine which conjugate base is HCOOH Ka = 1.7 x 10-4 (b) HCN Ka = 4.9 x 10-10arrow_forwardDraw all possible carboxylic acids with the formula C5H10O2.arrow_forwardA compound with empirical formula C2H5O was found in a separate experiment to have a molar mass of approximately 90 g. What is the molecular formula of the compound?arrow_forward
- Ethylene glycol, the main ingredient in antifreeze, contains 38.7% carbon, 9.7% hydrogen and 51.6 % oxygen. Calculate the empirical and molecular formulas for ethylene glycol. Given the molar mass is approximately 60 g/mol. A) Empirical formula: B)Molecular formula: Explain how you obtained the Molecular formula (b)?arrow_forwardDraw Lewis structures for two compounds of formula C2H7N.arrow_forwardGiven the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forward
- The reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardWrite the chemical formula of the conjugate base of boric acid.arrow_forwardGlutathione, a powerful antioxidant that destroys harmful oxidizing agents in cells, is composed of glutamic acid, cysteine, and glycine, and has the following structure. a.) What product is formed when glutathione reacts with an oxidizing agent?b.) What is unusual about the peptide bond between glutamic acid and cysteine?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





