
a)
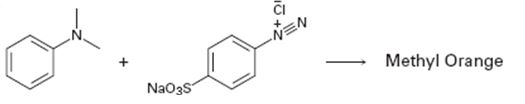
Interpretation:
A structure for methyl orange, an azo dye, produced when the two reactants shown react is to be drawn and the electron pushing mechanism for its formation is to be shown.
Concept introduction:
The diazonium cation can act as an electrophile and can attack
To draw:
The structure of methyl orange, an azo dye, produced when the two reactants shown react and to show the electron pushing mechanism for its formation.
b)
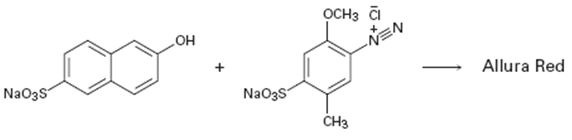
Interpretation:
A structure for allura red, an azo dye, produced when the two reactants shown react is to be drawn and the electron pushing mechanism for its formation is to be shown.
Concept introduction:
The diazonium cation can act as an electrophile and attack the aromatic rings. The dimethylamino group is an o- and p- directing group. Hence the diazonium cation can attack the ring at the p-position to yield a carbocation intermediate. The intermediate then can lose a proton to yield the desired product. This reaction is known as coupling reaction.
To draw:
The structure of allura red, an azo dye, produced when the two reactants shown react and to give the electron pushing mechanism for its formation.
c)
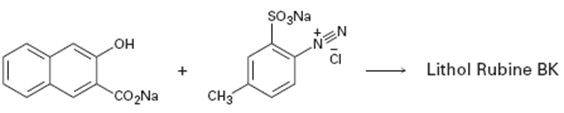
Interpretation:
A structure for lithol rubine BX, an azo dye, produced when the two reactants shown react is to be drawn and the electron pushing mechanism for its formation is to be shown.
Concept introduction:
The diazonium cation can act as an electrophile and attack the aromatic rings. The dimethylamino group is an o- and p- directing group. Hence the diazonium cation can attack the ring at the p-position to yield a carbocation intermediate. The intermediate then can lose a proton to yield the desired product. This reaction is known as coupling reaction.
To draw:
The structure of lithol rubine BX, an azo dye, produced when the two reactants shown react and to show the electron pushing mechanism for its formation.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forward
- One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardCompound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forwardOne step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forward
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardA graduate student tried to make o-fluorophenylmagnesium bromide by adding magnesium to an ether solution of o-fluorobromobenzene. After obtaining puzzling results with this reaction, she repeated the reaction by using as solvent some tetrahydrofuran that contained a small amount of furan. From this reaction, she isolated a fair yield of the compound that follows. Propose a mechanism for its formation.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


