
Composition diagrams, commonly known as “alpha plots,” are often used to visualize the species in a solution of an acid or base as the pH is varied. The diagram for 0.100 M acetic acid is shown here.
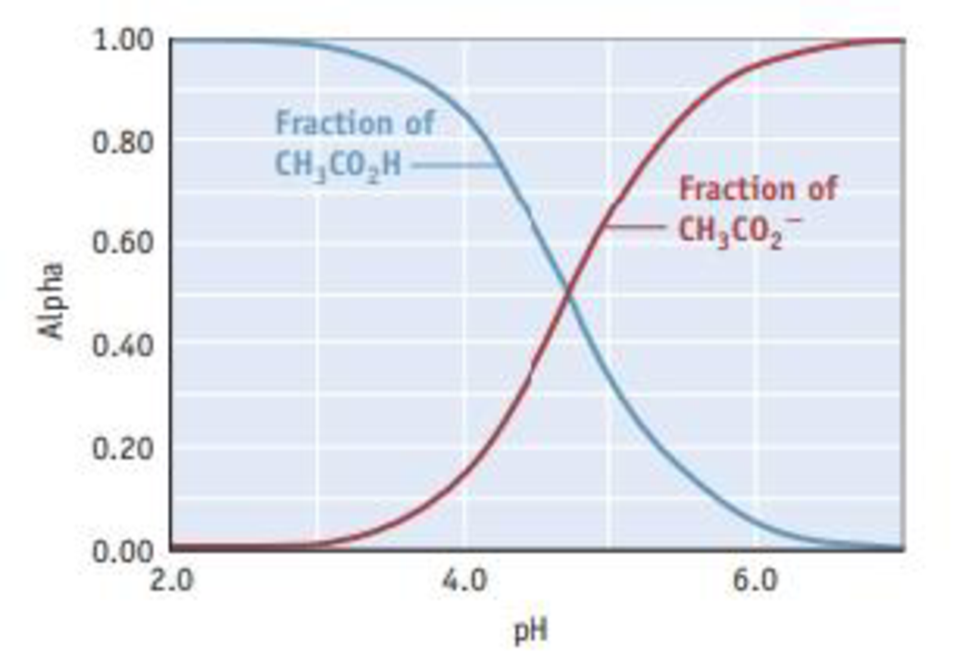
The plot shows how the fraction [alpha (α)] of acetic acid in solution,
changes as the pH increases (blue curve). (The red curve shows how the fraction of acetate ion, CH3CO2−, changes as the pH increases.) Alpha plots are another way of viewing the relative concentrations of acetic acid and acetate ion as a strong base is added to a solution of acetic acid in the course of a titration.
- (a) Explain why the fraction of acetic acid declines and that of acetate ion increases as the pH increases.
- (b) Which species predominates at a pH of 4, acetic acid or acetate ion? What is the situation at a pH of 6?
- (c) Consider the point where the two lines cross. The fraction of acetic acid in the solution is 0.5, and so is that of acetate ion. That is, the solution is half acid and half conjugate base; their concentrations are equal. At this point, the graph shows the pH is 4.74. Explain why the pH at this point is 4 74.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Chemistry & Chemical Reactivity
- Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H) and the amine group (NHR) are protonated. However, as the pH of the solution increases (say, by adding base), the carboxylic acid proton is removed, usually at a pH between 2 and 3. In a middle range of pHs, therefore, the amine group is protonated, but the carboxylic acid group has lost the proton. (This is called a zwitterion.) At more basic pH values, the amine proton is dissociated. What is the pH of a 0.20 M solution of alanine hydrochloride, [NH3CHCH3CO2H]Cl?arrow_forwardThe pigment cyanidin aglycone is one of the anthocyanin molecules that gives red cabbage (Brassica oleracea var. capitata f. rubra) its characteristic red coloration. Many chemistry students have used this red cabbage indicator to study acid-base chemistry. Estimate tire pH range at which cyanidin agly-cone shows a color change. Anth-H(aq) Anth(aq) + H+ (aq) Ka = 1.3 107arrow_forwardMalic acid is a weak diprotic organic acid with Ka1 = 4.0 104 and Ka2 = 9.0 105. a Letting the symbol H2A represent malic acid, write the chemical equations that represent Ka1 and Ka2. Write the chemical equation that represents Ka1 Ka2. b Qualitatively describe the relative concentrations of H2A, HA, A2, and H3O+ in a solution that is about one molar in malic acid. c Calculate the pH of a 0.0175 M malic acid solution and the equilibrium concentration of [H2A]. d What is the A2 concentrationin in solutions b and c?arrow_forward
- The simplest amino acid is glycine, H2NCH2CO2H. The common feature of amino acids is that they contain the functional groups: an amine group, -NH2, and a carboxylic acid group, -CO2H. An amino acid can function as either an acid or a base. For glycine, the acid strength of the carboxyl group is about the same as that of acetic acid. CH3CO2H, and the base strength of the amino group is slightly greater than that of ammonia, NH3. (a) Write the Lewis structures of the ions that form when glycine is dissolved in 1 M HCl and in 1 M KOH. (b) Write the Lewis structure of glycine when this amino acid is dissolved in water. (Hint: Consider the relative base strengths of the -NH2 and -CO2- groups.)arrow_forwardSketch a titration curve for the titration of potassium hydroxide with HCl, both 0.100 M. Identify three regions in which a particular chemical species or system dominates the acid-base equilibria.arrow_forwardConsider all acid-base indicators discussed in this chapter. Which of these indicators would be suitable for the titration of each of these? (a) NaOH with HClO4 (b) acetic acid with KOH (c) NH3 solution with HBr (d) KOH with HNO3 Explain your choices.arrow_forward
- For conjugate acidbase pairs, how are Ka and Kb related? Consider the reaction of acetic acid in water CH3CO2H(aq)+H2O(l)CH3CO2(aq)+H3O+(aq) where Ka = 1.8 105 a. Which two bases are competing for the proton? b. Which is the stronger base? c. In light of your answer to part b. why do we classify the acetate ion (CH3CO2) as a weak base? Use an appropriate reaction to justify your answer. In general, as base strength increases, conjugate acid strength decreases. Explain why the conjugate acid of the weak base NH3 is a weak acid. To summarize, the conjugate base of a weak acid is a weak base and the conjugate acid of a weak base is a weak acid (weak gives you weak). Assuming Ka for a monoprotic strong acid is 1 106, calculate Kb for the conjugate base of this strong acid. Why do conjugate bases of strong acids have no basic properties in water? List the conjugate bases of the six common strong acids. To tie it all together, some instructors have students think of Li+, K+, Rb+, Cs+, Ca2+, Sr2+, and Ba2+ as the conjugate acids of the strong bases LiOH, KOH. RbOH, CsOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2. Although not technically correct, the conjugate acid strength of these cations is similar to the conjugate base strength of the strong acids. That is, these cations have no acidic properties in water; similarly, the conjugate bases of strong acids have no basic properties (strong gives you worthless). Fill in the blanks with the correct response. The conjugate base of a weak acid is a_____base. The conjugate acid of a weak base is a_____acid. The conjugate base of a strong acid is a_____base. The conjugate acid of a strong base is a_____ acid. (Hint: Weak gives you weak and strong gives you worthless.)arrow_forwardWhat is the pH of a solution prepared by combining 0.258 mol of a newly discovered weak acid (Ka = 1.3 x 10-4) with 0.156 mol of the salt containing its conjugate base in 1.4 L of water?arrow_forwardWhat is the pH of a solution prepared by mixing 50.00 mL of 0.10 M methylamine, CH 3NH 2, with 25.00 mL of 0.10 M methylammonium chloride, CH 3NH 3Cl? Assume that the volume of the solutions are additive and that K b = 3.70 × 10 -4 for methylaminearrow_forward
- Calculate the pH of a solution prepared by mixing 15.0 mL of .100 M NaOH and 30 mL of .100 M benzoic acid solution. (Benzoic acid is monoprotic; its acid dissociation constant is 6.46 x 10^-5.)?arrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.458 M aqueous solution of dimethylamine, (CH3)2NH to be 12.230. Use the information she obtained to determine the Kb for this base. Kb(experiment) =arrow_forwardCalculate the pH of a solution made by dissolving 1.87g of sodium caproate (NaC6H11O2) in water and diluting it toa total volume of 500mL. For the caproate ion, Kb=7.58 x 10^-10arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





