
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 119SCQ
The chemical name for aspirin is acetylsalicylic acid. It is believed that the analgesic and other desirable properties of aspirin are due not to the aspirin itself but rather to the simpler compound salicylic add, C6H4(OH)CO2H, which results from the breakdown of asp inn in the stomach.
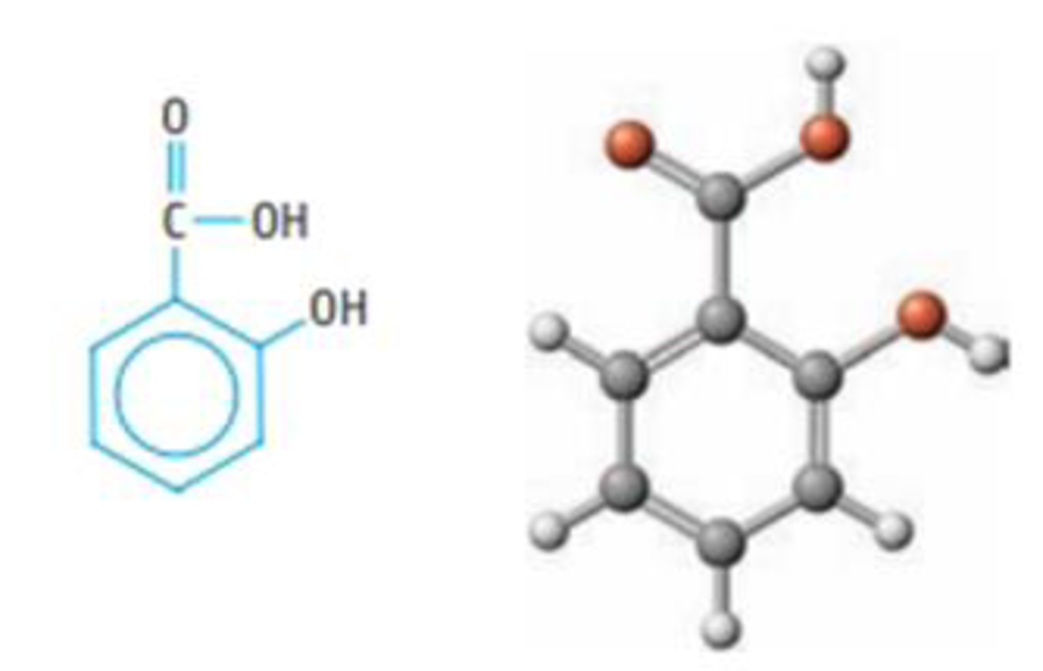
Salicylic acid
- (a) Give approximate values for the following bond angles in the acid: (i) C—C—C in the ring, (ii) O—C=O; (iii) either of the C—O—H angles: and (iv) C—C—H.
- (b) What is the hybridization of the C atoms of the ring? Of the C atom in the —CO2H group?
- (c) Experiment shows that 1.00 g of the acid will dissolve in 460 ml of water. If the pH of this solution is 2.4, what is Ka for the acid?
- (d) If you have salicylic acid in your stomach and if the pH of gastric juice is 2.0, calculate the percentage of salicylic add that will be present in the stomach in the form of the salicylate ion, C6H4(OH)CO2−.
- (e) Assume you have 25.0 mL of a 0.014 M solution of salicylic acid and titrate it with 0.010 M NaOH. What is the pH at the halfway point of the titration? What is the pH at the equivalence point?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The pH of an aqueous solution of 0.522 M benzoic acid, C6H5COOH is
The pH value of the 5L aqueous solution prepared with 6.4 grams of a precious strong acid is 2. So what is the molar mass of the acid?
what is the change in enthalpy in kJ for the neutralization reaction between HCl and NaOH?
Chapter 17 Solutions
Chemistry & Chemical Reactivity
Ch. 17.1 - You have a 0.30 M solution of formic acid (HCO2H)...Ch. 17.2 - What is the pH of a buffer solution composed of...Ch. 17.2 - Use the Henderson-Hasselbalch equation to...Ch. 17.2 - Using an acetic acid/sodium acetate buffer...Ch. 17.2 - Calculate the pH of 0.500 L of a buffer solution...Ch. 17.3 - The titration of 0.100 M acetic acid with 0.100 M...Ch. 17.3 - Calculate the pH after 75.0 mL of 0.100 M HO has...Ch. 17.4 - The barium ion concentration, [Ba2+], in a...Ch. 17.4 - Calculate the solubility of AgCN in moles per...Ch. 17.4 - Calculate the solubility of Ca(OH)2 in moles per...
Ch. 17.4 - Calculate the solubility of BaSO4 (a) in pure...Ch. 17.5 - Solid Pbl2 (Ksp = 9.8 109) is placed in a beaker...Ch. 17.5 - Prob. 17.13CYUCh. 17.5 - Prob. 17.14CYUCh. 17.6 - Silver nitrate (0.0050 mol) is added to 1.00 L of...Ch. 17.6 - Calculate the value of the equilibrium constant,...Ch. 17.6 - Prob. 1.1ACPCh. 17.6 - What is the minimum volume of 0.0071 M NaCN(aq)...Ch. 17.6 - Use the formation constant of [Au(CN)2] in...Ch. 17.6 - Silver undergoes similar reactions as those shown...Ch. 17.6 - Write a balanced chemical equation for the...Ch. 17.6 - Phosphate ions are abundant in cells, both as the...Ch. 17.6 - A typical total phosphate concentration in a cell,...Ch. 17 - Does the pH of the solution increase, decrease or...Ch. 17 - Does the pH of the solution increase, decrease, or...Ch. 17 - What is the pH of a solution that consists of 0.20...Ch. 17 - What is the pH of 0.15 M acetic acid to which 1.56...Ch. 17 - What is the pH of the solution that results from...Ch. 17 - What is the pH of the solution that results from...Ch. 17 - What is the pH of the buffer solution that...Ch. 17 - Lactic acid (CH3CHOHCO2H) is found in sour milk,...Ch. 17 - What mass of sodium acetate, NaCH3CO2, must he...Ch. 17 - What mass of ammonium chloride, NH4Cl, must be...Ch. 17 - Calculate the pH of a solution that has an acetic...Ch. 17 - Calculate the pH of a solution that has an...Ch. 17 - What must the ratio of acetic acid to acetate ion...Ch. 17 - What must the ratio of H2PO4 to HPO42 be to have a...Ch. 17 - A buffer is composed of formic acid and its...Ch. 17 - A buffer solution is composed of 1.360 g of KH2PO4...Ch. 17 - Which of the following combinations would be the...Ch. 17 - Which of the following combinations would be the...Ch. 17 - Describe how to prepare a buffer solution from...Ch. 17 - Describe how to prepare a buffer solution from NH3...Ch. 17 - Determine the volume (in mL) of 1.00 M NaOH that...Ch. 17 - Determine the volume (in mL) of 1.00 M HC1 that...Ch. 17 - A buffer solution was prepared by adding 4.95 g of...Ch. 17 - You dissolve 0.425 g of NaOH in 2.00 L of a buffer...Ch. 17 - A buffer solution is prepared by adding 0.125 mol...Ch. 17 - What is the pH change when 20.0 mL of 0.100 M NaOH...Ch. 17 - Phenol, C6H5OH, is a weak organic acid. Suppose...Ch. 17 - Assume you dissolve 0.235 g of the weak acid...Ch. 17 - You require 36.78 mL of 0.0105 M HCl to reach the...Ch. 17 - A titration of 25.0 mL of a solution of the weak...Ch. 17 - Without doing detailed calculations, sketch the...Ch. 17 - Without doing detailed calculations, sketch the...Ch. 17 - You titrate 25.0 mL of 0.10 M NH3 with 0.10 M HCl....Ch. 17 - Using Figure 17.11, suggest an indicator to use in...Ch. 17 - Using Figure 17.11, suggest an indicator to use in...Ch. 17 - Name two insoluble salts of each of the following...Ch. 17 - Prob. 38PSCh. 17 - Using the solubility guidelines (Figure 3.10),...Ch. 17 - Predict whether each of the fallowing is insoluble...Ch. 17 - For each of the following insoluble salts, (1)...Ch. 17 - Prob. 42PSCh. 17 - When 1.55 g of solid thallium(I) bromide is added...Ch. 17 - At 20 C, a saturated aqueous solution of silver...Ch. 17 - When 250 mg of SrF2, strontium fluoride, is added...Ch. 17 - Calcium hydroxide, Ca(OH)2, dissolves in water to...Ch. 17 - You add 0.979 g of Pb(OH)2 to 1.00 L of pure water...Ch. 17 - You place 1.234 g of solid Ca(OH)2 in 1.00 L of...Ch. 17 - Estimate the solubility of silver iodide in pure...Ch. 17 - What is the molar concentration of Au+(aq) in a...Ch. 17 - Prob. 51PSCh. 17 - Estimate the solubility of lead(II) bromide (a) in...Ch. 17 - The Ksp value for radium sulfate, RaSO4, is 4.2 ...Ch. 17 - If 55 mg of lead(II) sulfate is placed in 250 mL...Ch. 17 - Prob. 55PSCh. 17 - Prob. 56PSCh. 17 - Calculate the molar solubility of silver...Ch. 17 - Calculate the solubility of silver bromide, AgBr,...Ch. 17 - Compare the solubility, in milligrams per...Ch. 17 - What is the solubility, in milligrams per...Ch. 17 - Calculate the solubility, in moles per liter, of...Ch. 17 - Calculate the solubility, in moles per liter, of...Ch. 17 - Which insoluble compound in each pair should be...Ch. 17 - Which compound in each pair is more soluble in...Ch. 17 - You have a solution that has a lead(II) ion...Ch. 17 - Sodium carbonate is added to a solution in which...Ch. 17 - If the concentration of Zn2+ in 10.0 mL of water...Ch. 17 - You have 95 mL of a solution that has a lead(II)...Ch. 17 - Prob. 69PSCh. 17 - Will a precipitate of Mg(OH)2 form when 25.0 mL of...Ch. 17 - Zinc hydroxide is amphoteric (Section 16.10). Use...Ch. 17 - Solid silver iodide, AgI, can be dissolved by...Ch. 17 - What amount of ammonia (moles) must be added to...Ch. 17 - Can you dissolve 15.0 mg of AuCl in 100.0 mL of...Ch. 17 - What is the solubility of AgCl (a) in pure water...Ch. 17 - Prob. 76PSCh. 17 - Prob. 77GQCh. 17 - Prob. 78GQCh. 17 - Prob. 79GQCh. 17 - Calculate the hydronium ion concentration and the...Ch. 17 - Calculate the hydronium ion concentration and the...Ch. 17 - For each of the following cases, decide whether...Ch. 17 - Prob. 83GQCh. 17 - A sample of hard water contains about 2.0 103 M...Ch. 17 - What is the pH of a buffer solution prepared from...Ch. 17 - Prob. 86GQCh. 17 - Describe the effect on the pH of the following...Ch. 17 - What volume of 0.120 M NaOH must be added to 100....Ch. 17 - A buffer solution is prepared by dissolving 1.50 g...Ch. 17 - What volume of 0.200 M HCl must be added to 500.0...Ch. 17 - What is the equilibrium constant for the following...Ch. 17 - Calculate the equilibrium constant for the...Ch. 17 - Prob. 93GQCh. 17 - The solubility product constant for calcium...Ch. 17 - In principle, the ions Ba2+ and Ca2+ can be...Ch. 17 - A solution contains 0.10 M iodide ion, I, and 0.10...Ch. 17 - A solution contains Ca2+ and Pb2+ ions, both at a...Ch. 17 - Prob. 98GQCh. 17 - Prob. 99GQCh. 17 - Prob. 100GQCh. 17 - Each pair of ions below is found together in...Ch. 17 - Each pair of ions below is found together in...Ch. 17 - The cations Ba2+ and Sr2+ can be precipitated as...Ch. 17 - You will often work with salts of Fe3+, Pb2+, and...Ch. 17 - Aniline hydrochloride, (C6H5NH3)Cl, is a weak...Ch. 17 - The weak base ethanolamine. HOCH2CH2NH2, can be...Ch. 17 - For the titration of 50.0 mL of 0.150 M...Ch. 17 - A buffer solution with it pH of 12.00 consists of...Ch. 17 - To have a buffer with a pH of 2.50, what volume of...Ch. 17 - What mass of Na3PO4 must be added to 80.0 mL of...Ch. 17 - You have a solution that contains AgNO3, Pb(NO3)2,...Ch. 17 - Prob. 112ILCh. 17 - Suggest a method for separating a precipitate...Ch. 17 - Prob. 114SCQCh. 17 - Prob. 115SCQCh. 17 - Two acids, each approximately 0.01 M in...Ch. 17 - Composition diagrams, commonly known as alpha...Ch. 17 - The composition diagram, or alpha plot, for the...Ch. 17 - The chemical name for aspirin is acetylsalicylic...Ch. 17 - Prob. 120SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Discuss the justification for this statement: “Although one does not normally regard NH+4 as an acid, it is actually only slightly weaker as an acid than hydrocyanic acid, HCN, in aqueous solution.”arrow_forwardPredict the position of equilibrium and calculate the equilibrium constant, Keq, for acid-base reactionarrow_forwardThe pH of an aqueous solution of acetic acid (CH3COOH) is 2.0. What is the initial molar concentration of CH3COOH, if its acid ionization constant is Ka = 1.8×10–5?arrow_forward
- Arrange the Lewis acids GaCl3, BCl3 en AlCl3 in order of increasing acidity.arrow_forwardUsing paleolimnological methods it is possible to estimate past pH values of surface waters. If the estimate of pH is 5.787 , what is the corresponding PCO2? in atm.arrow_forwardWhat is the degree of ionization (i) for H₂SO₄(aq)?arrow_forward
- Calculate the enthalpy of reaction of the following dissolution: NaOH(s)+aq=Na(aq)+OH^- NaHCO3(s)+aq=Na(aq)+HCO3^-arrow_forward6-78 (Chemical Connections 6A) Oxides of nitrogen (NO, NO2,N2O3) are also responsible for acid rain. Which acids can be formed from these nitrogen oxides?arrow_forwardLeucine is an amino acid with this Lewis structure: Write the Lewis structure for the zwitterion form of leucine.arrow_forward
- The pH of an aqueous solution of 0.458 M pyridine (a weak base with the formula C5H5N) is ?arrow_forwardThe pH of an aqueous solution of 3.14×10-2 M ammonium chloride, NH4Cl (aq), isarrow_forwardSuppose that, instead of using NaOH, a base such as Ba(OH)2 had been used. What changes in the calculations would then have to be made to determine the molar concentrations of the base?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning  Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY