
Concept explainers
17-19 Write the JUPAC names for these compounds.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
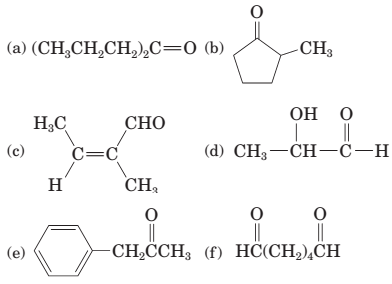
The given structure is:
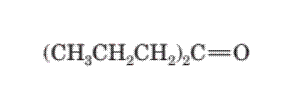
The parent chain in the given structure is heptane. Numbering is done in such a way that carbonyl carbon gets number 4.

So, the IUPAC name will be: heptanes-4-one.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
The given structure is:
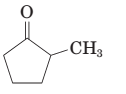
The parent chain in the given structure is cyclopentane and methyl group is substituted at second carbon.
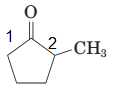
So, the IUPAC name will be: 2-methylcyclopentanone.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
The given structure is:
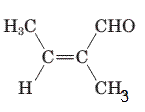
The parent chain in the given structure is butene. Numbering is done in such a way that aldehyde group gets smallest number.
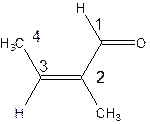
So, the IUPAC name will be: 2-methylbut-2-enal.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
The given structure is:
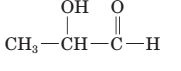
The parent chain in the given structure is propane. Numbering is done in such a way that aldehyde group gets smallest number and −OH group on carbon number second will behave as substituent.
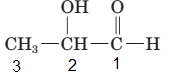
So, the IUPAC name will be: 2-hydroxypropanal.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest)alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
The given structure is:
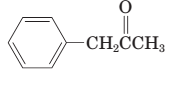
The parent chain in the given structure is propane. Numbering is done in such a way that ketone group gets smallest number and benzene ring on carbon number first will behave as substituent.
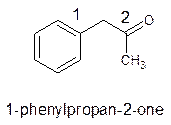
So, the IUPAC name will be: 1-phenylpropan-2-one.
Interpretation:
The IUPAC names of the given compound should be determined.
Concept Introduction:
The group that contains carbonyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carbonyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the IUPAC name to the aldehyde group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
In order to give the IUPAC name to the ketone group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
Explanation of Solution
The given structure is:
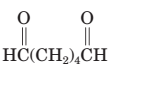
The parent chain in the given structure is hexane. Numbering is done in such a way that aldehyde group gets smallest number.
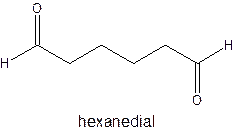
So, the IUPAC name will be: hexanedial.
Want to see more full solutions like this?
Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
- 18-15 Draw a structural formula for the dimer formed when two molecules of formic acid interact by hydrogen bonding.arrow_forward18-19 The following compounds have approximately the same molecular weight: hexanoic acid, heptanal, and 1-heptanol. Arrange them in order of increasing boiling point.arrow_forward18-17 Hexanoic (caproic) acid has a solubility in water of about 1 g/100 mL water. Which part of the molecule contributes to water solubility, and which part prevents solubility?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
