
Concept explainers
(a)
Interpretation:
The number of carbon‑nitrogen bonds present in primary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the
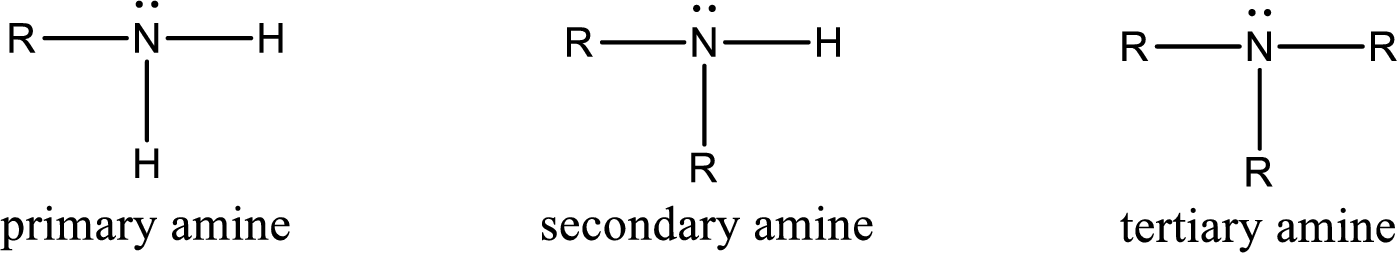
(b)
Interpretation:
The number of carbon‑nitrogen bonds present in secondary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
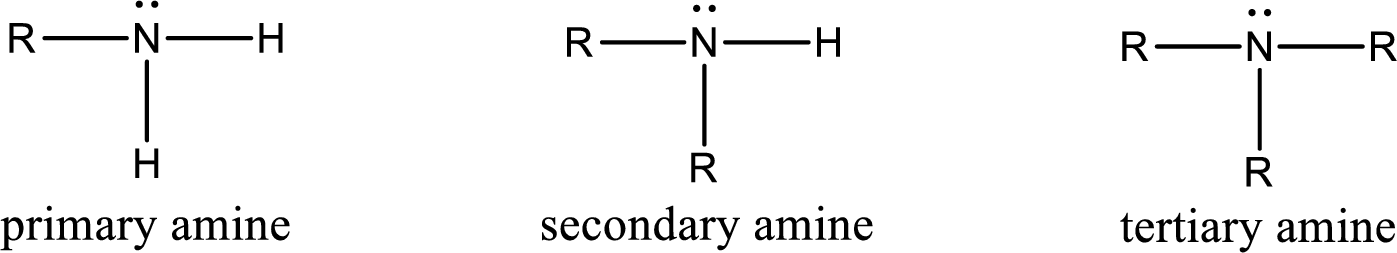
(c)
Interpretation:
The number of carbon‑nitrogen bonds present in tertiary amine has to be given.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
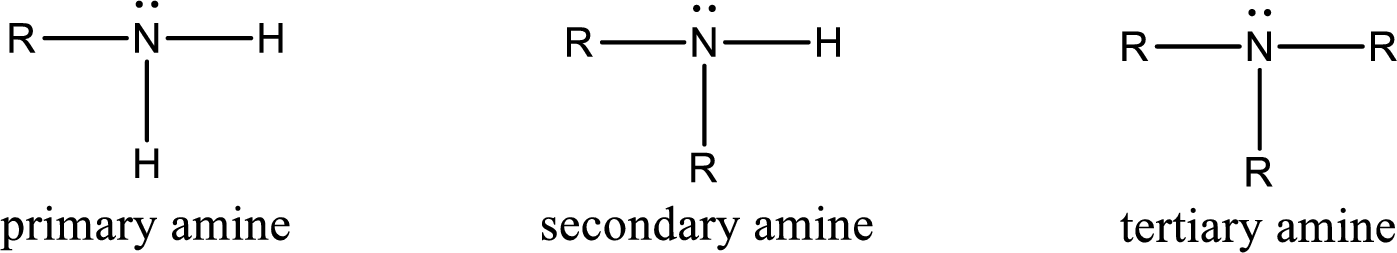
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, and Biological Chemistry
- amines Name and classify each compoundarrow_forwardPrimary and secondary amines _____ dissolve well in nonpolar solvents, such as acetone react readily with each other All of the given are correct form hydrogen bonds when dissolved in waterarrow_forwardWhat is the generic structure of amines? Write the structures of two specific amines.arrow_forward
- Which of the following statements is true for an amine if "N-" is part of the IUPAC name? a. The compound is a primary amine. b. The molecule is contains a nitrogen atom attached to carbon number one. c. The compound is a secondary amine. d. The compound is a tertiary amine.arrow_forwardIdentify the characteristics of aminesarrow_forwardDraw a structural formula for each amine and amine derivative. Q.) tert-Butylaminearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning




